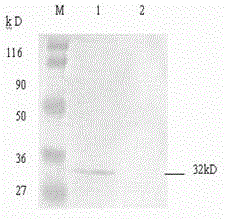
Koi herpesvirus ORF25 nucleic acid vaccine
A koi herpes virus, ORF25 technology, applied in the directions of viral peptides, antiviral agents, biochemical equipment and methods, etc., can solve the death of carp, the blow of carp breeding industry, and the inability of koi herpes virus disease to play a complete immune protection effect and other problems, to achieve the effect of small immune dose, maintenance of ecological stability, and reduction of drug use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Cloning and identification of ORF25 gene
[0022] 1. Design ORF25 gene amplification primers
[0023] According to the KHV-I strain ORF25 gene sequence registered on GenBank, primers were designed using the software Primer Premiers 5.0, and the upstream primer was: F1 5′- GATATC ATGACGGGTTGTGGGGTT-3′, the downstream primer is: F2 5′- GAATTC TTAGGGCCTCCGGGA-3', two restriction enzyme cutting sites (underlined sites) of EcoR V and EcoR I were respectively introduced, and the primers were synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.
[0024] 2. PCR amplification and identification of ORF25 gene
[0025] Using KHV nucleic acid DNA as a template, the ORF25 gene was amplified by PCR. The reaction system is: F1, F2 (20pmol / μL) 0.5μL, 10×ExBuffer 2.5μL, dNTP 2μL, DNA 1μL, ExTaq enzyme 0.25μL, add sterilized water to make up to 25μL. The PCR reaction conditions were: pre-denaturation at 95°C, 5min; denaturation at 94°C, 40s; anne...
Embodiment 2
[0027] Example 2: Construction and identification of pIRES-ORF25 recombinant eukaryotic expression plasmid
[0028] 1. Preparation of pIRES-neo plasmid
[0029] Take 5 μl of DH5α bacteria liquid containing pIRES-neo plasmid (purchased from Kingvik (China) Biotechnology Center), add it to 5 mL of LB liquid medium containing ampicillin, and culture overnight at 37°C with shaking at 180r / min. A small amount of plasmids were prepared according to the SDS alkaline lysis method of the Molecular Cloning Operation Guide (Third Edition).
[0030] 2. Digestion of pIRES-neo plasmid
[0031] The pIRES-neo plasmid was subjected to EcoR V and EcoR I double enzyme digestion, 1.0% agarose electrophoresis, and a large piece of enzyme digestion was recovered according to the instructions of the Tiangen company's agarose gel DNA recovery kit.
[0032] 3. Cloning of ORF25 gene in pIRES-neo plasmid
[0033]Use Tiangen Company Agarose Gel DNA Recovery Kit to recover and purify the PCR product of...
Embodiment 3
[0037] Example 3: Expression of recombinant plasmid pIRES-ORF25 in MFC cells
[0038] 1. Mass extraction and purification of recombinant plasmids
[0039] Transform the identified positive pIRES-ORF25 plasmid into Escherichia coli DH5α competent cells, pick a single colony; inoculate it in 5 mL of LB liquid culture medium containing Amp+, culture with shaking at 37°C until the logarithmic growth phase (OD600≈0.6); Take 1mL and inoculate it into 500mL LB liquid culture solution containing Amp to expand the culture. Centrifuge the above culture at 5000r / min for 10min, discard the supernatant, place it in a low-temperature freezer for several hours, and use 200ml ice-precooled STE (0.1mol / L NaCl, 10mmol / L Tris-HCl pH8.0, 1mmol / L EDTA ) to resuspend the bacterial pellet, and collect the bacteria by centrifugation as described above.
[0040] Refer to the Molecular Cloning Operation Guide (Third Edition) SDS alkaline lysis method to prepare a large number of plasmids and extract ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com