Porcine epidemic diarrhea live vaccine
A technology for porcine epidemic diarrhea and vaccines, which is applied to medical preparations with non-active ingredients, digestive system, non-active ingredients of polymer compounds, etc., can solve the problems of unsatisfactory clinical application effect, etc. Good, high antibody titer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1: Screening of porcine epidemic diarrhea virus SD10 strain
[0012] 1.1 Isolation and Identification of Porcine Epidemic Diarrhea Virus SD10 Strain During the epidemiological investigation, a strain of porcine epidemic diarrhea virus (named SD10A1 strain) was isolated from the contents of the small intestine of a pig farm with diarrhea in Laixi, Shandong Province. ), porcine epidemic diarrhea virus RT-PCR test was positive, blind transmission to the 8th generation of cytopathy, the 16th generation of regular and stable cytopathy, virus content ≥ 10 5.0 TCID 50 / 0.1ml, can be neutralized by anti-porcine epidemic diarrhea virus-specific serum. When passed to the 121st generation, the passaged virus is safe, pure, and free of bacteria, mold, and mycoplasma for 3-5 day-old healthy piglets that have not fed colostrum and exogenous viruses (classic swine fever, porcine parvovirus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome, porcine fo...
Embodiment 2
[0038] The safety test of embodiment 2 porcine epidemic diarrhea live vaccine
[0039] 1. Materials and methods
[0040] 1.1 Single dose test
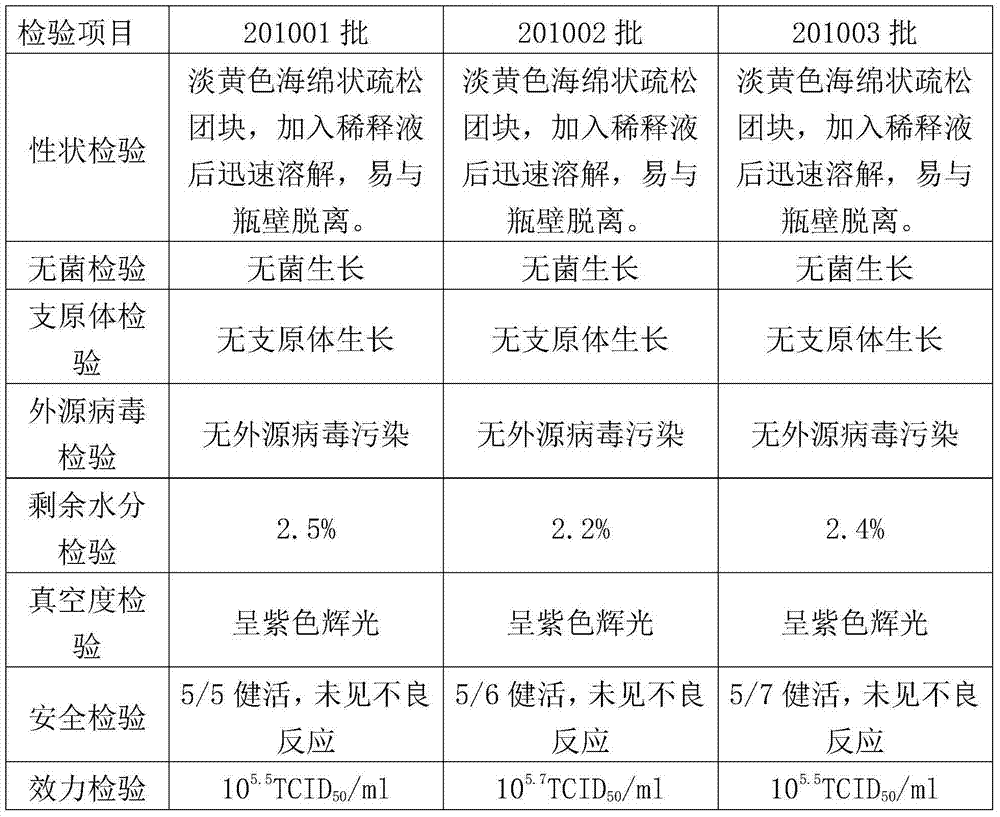
[0041] 1.1.1 Single-dose test on pregnant sows Take 6 pregnant sows that were negative for porcine epidemic diarrhea neutralizing antibodies and antigens 4 to 6 weeks before delivery, and randomly divided them into 2 groups, 3 in each group, the first group The 201001 batches of porcine epidemic diarrhea live vaccines were intramuscularly injected with 1 part per head, and the second group was not injected as the control group, and the sows were observed until the sows gave birth.
[0042] 1.1.2 Single-dose test on piglets Take 12 1-day-old piglets and randomly divide them into 2 groups, 6 pigs in each group. The first group takes 1 batch of 201001 porcine epidemic diarrhea live vaccine orally, and the second group does not Oral as a control group, observed for 14 days.
[0043] 1.2 Single-dose repeated test
[0044]1.2.1 Repeated ...
Embodiment 3
[0053] Efficacy test of embodiment 3 porcine epidemic diarrhea live vaccine
[0054] 1. Active immunization test
[0055] 1.1 Materials and methods Eight sows were randomly divided into 2 groups with 4 pigs in each group. The first group was injected with 201,001 batches of porcine epidemic diarrhea live vaccine in the neck muscle, and the second group was not injected as a control. Group, after the sows gave birth, 14 piglets were taken respectively at the age of 3 days, 7 days, 14 days, and 21 days, and were randomly divided into 2 groups, with 7 pigs in each group. Clinical manifestations of piglets after challenge.
[0056] 1.2 Results The piglets produced by the immunized sows, after the 3-day-old challenge, 7 immunized piglets showed no abnormalities in lactation, spirit, and feces, and were all healthy and alive; after the 7 control piglets were challenged, 7 / 7 showed typical pig epidemics. Sexual diarrhea symptoms, 6 / 7 died. After the 7-day-old challenge, the immune...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com