High immunogenicity rabies virus glycoprotein, its preparation method and application
A technology of rabies virus and high immunity, which is applied in the field of 0., the preparation of highly immunogenic rabies virus glycoprotein, and the field of highly immunogenic rabies virus glycoprotein, to reduce production costs, avoid vaccine failure, and improve immunogenicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Artificial Synthesis of Highly Immunogenic Rabies Virus Glycoprotein Gene
[0030] The gene shown in SEQ No.1 is divided into two parts, the A fragment of 1-730bp and the B fragment of 710-1575bp, and TaKaRa (Dalian) Company is entrusted to carry out artificial synthesis respectively, and the synthesized A and B gene fragments are cloned into The BamHI site of the pUC18 plasmid vector was transformed into Escherichia coli JM109 competent, and the recombinant plasmid obtained by screening was positively connected, that is, the upstream of the fragment was close to the HindIII site and the downstream was close to the EcoRI site, and the SphI site at the 721bp position of the sequence could be used as the next One-step gene cloning. The new plasmid was named pUC18-A ( Figure 4 ) and pUC18-B ( Figure 5 ), the A and B sequence fragments in the plasmid have been sequenced and are completely consistent with the DNA sequence shown in SEQ No1.
Embodiment 2
[0032] Construction of recombinant adenovirus with highly immunogenic rabies virus glycoprotein
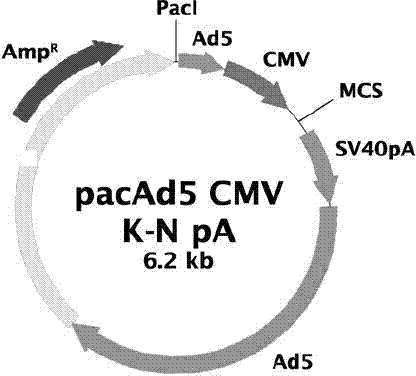
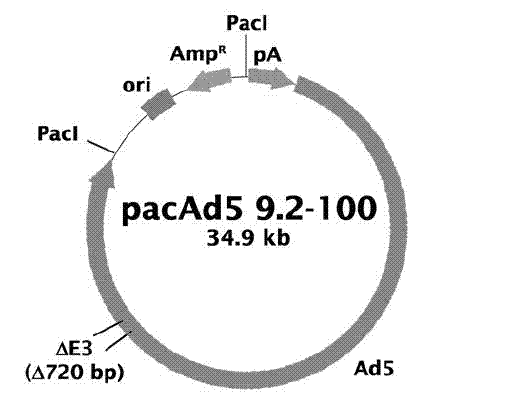
[0033] Plasmid pUC18-A was double-digested with restriction endonucleases HindIII and SphI, and a 730bp A fragment was recovered, and pUC18-B was double-digested with SphI and EcoRI, and an 870bp B fragment was recovered. Then, the adenoviral shuttle plasmid pacAd5 CMV was double-digested with HindIII and EcoRI, and a 6.2kb vector fragment was recovered. The carrier fragment was connected with the two gene fragments A and B under the action of T4 DNA ligase, transformed into Escherichia coli JM109 competent, and the recombinant plasmid pacAd5 CMV-optG ( Figure 6 ). Plasmids pacAd5 CMV-optG and pacAd5 9.2-100, 100 μg each, were mixed with liposomes (Invitrogen lipofectamine 2000) and then transfected into 293AD monolayer cells. The culture was continued for 5 days, and the cell culture was harvested until obvious cells became round and aggregated into bunches of grapes. The c...
Embodiment 3
[0035] Preparation of recombinant adenovirus expressing highly immunogenic rabies virus glycoprotein
[0036] The recombinant adenovirus rAD5-optG seed virus obtained in Example 2 was inoculated with 5% volume of 293AD monolayer cells, 37 ° C, 5% CO 2 Cultivate under conditions for 4-6 days, and harvest the culture by freezing and thawing when the cells become obviously round and aggregate into grape bunches. After harvesting, the virus can be expanded and inoculated according to this method, and a large amount of recombinant adenovirus rAD5-optG can be prepared.
[0037] Take the recombinant virus prepared in each batch, press 1, 10, 10 2 、10 3 、10 4 ...10 11 Perform gradient dilution, inoculate 293AD monolayer cells in a 96-well plate, inoculate 8 replicate wells for each gradient, and use 100 μl of culture medium in each well. 37°C, 5% CO after inoculation 2 Cultured under conditions for 7 days, observe and record whether there is cytopathy in each well. Recombinan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com