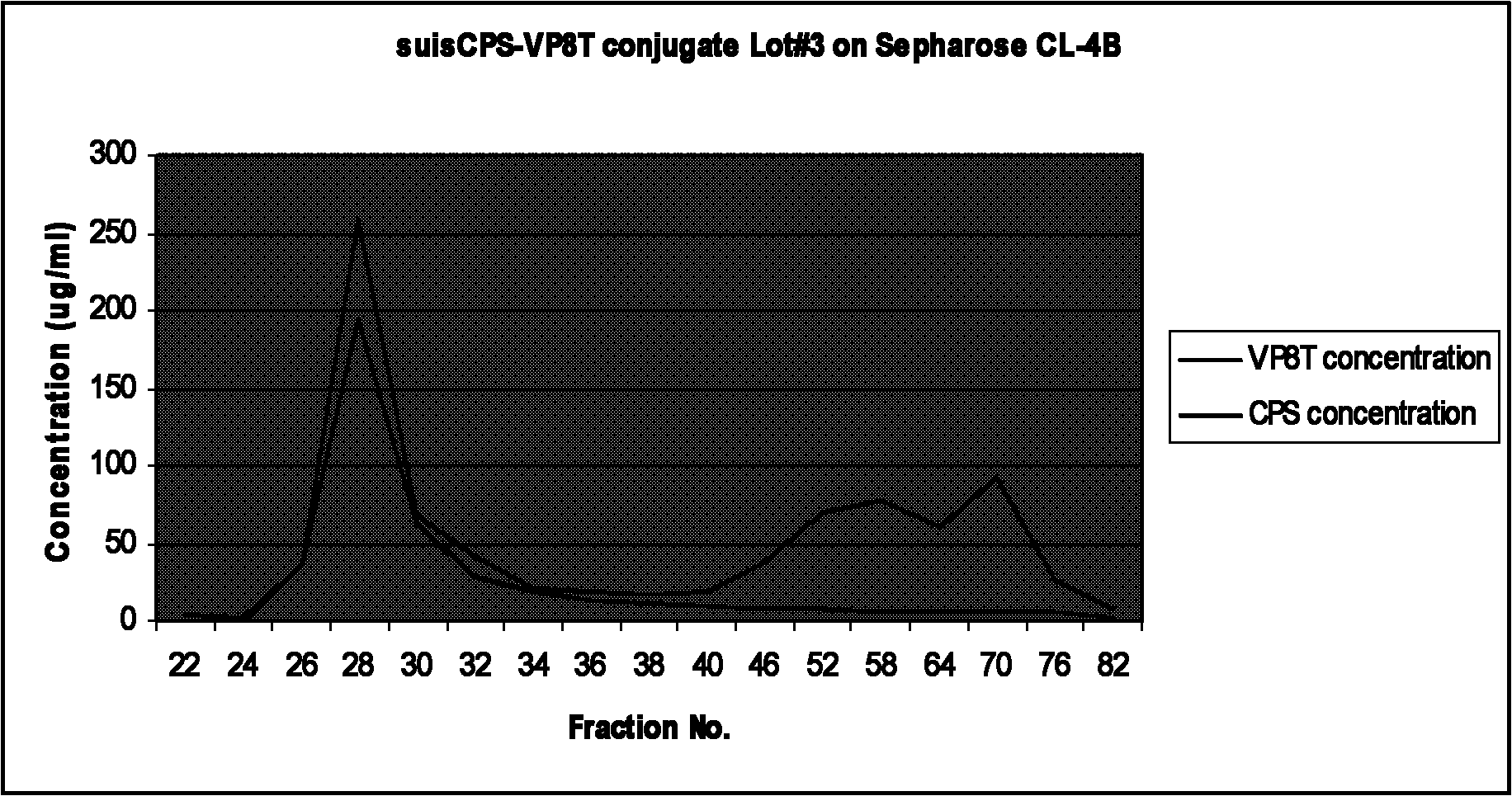
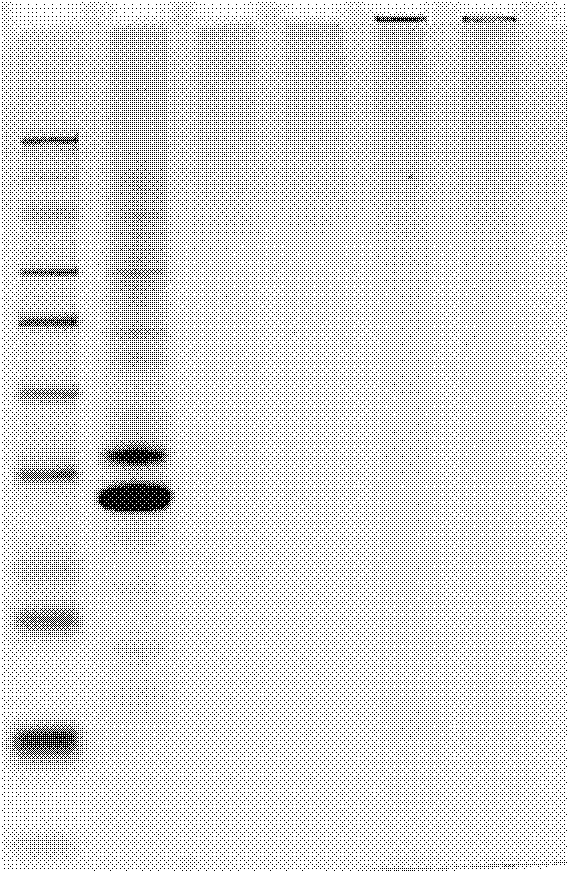Bacterial polysaccharide-protein conjugate vaccine and preparation method thereof
A protein-conjugated vaccine, bacterial polysaccharide technology, applied in the field of conjugated vaccines, can solve problems such as lack of protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1 Establishment of cDNA library of rotavirus
[0128] Human rotavirus Wa strain (2018-VR, ATCC) was amplified at 36°C for 1 to 2 days in MA104 cells (monkey embryonic kidney cells) supplemented with 1 μg / ml trypsin. Cell debris was removed by low-speed centrifugation, and hydroxyapatite (HA) was added to the supernatant to purify viral RNA. The specific steps are as follows: 600 μl of 6M guanidine isothiocyanate (GITC) solution is added to 400 μl of culture supernatant, and mixed. 50 [mu]l (two drops) of HA suspension was then added and the tube was mixed by inversion upside down or on a shaker for 20 minutes at room temperature to allow adsorption of nucleic acids to HA. Centrifuge at 13,000 g for 1 min and discard the supernatant. Wash RNA-HA twice with 1 ml of 10 mM potassium phosphate pH 6.8. Add 40 μl of 200 mM potassium phosphate pH6.8 solution to elute RNA from HA crystals, and collect purified RNA by centrifugation at 13000 g for 3 minutes. Perform r...
Embodiment 2
[0134] Example 2 Construction of pET28aWaVP8 expression full-length VP8 plasmid
[0135] The pGEM-TWaVP8 prepared in Example 1 was digested with restriction endonucleases NdeI and BamHI to obtain the insertion sequence of the VP8 gene. The vector pET28a(+) was also digested with restriction endonucleases NdeI and BamHI. Add 10 μg of VP8 gene insertion sequence, T4 DNA ligase and cut pET28a(+) to the test tube, and react overnight at 16°C. The constructed plasmid was called pET28aWaVP8. The protein expressed by the plasmid contains a 6-histidine tag (hexa-histidine tag, 6×His-Tag), and a thrombin recognition site (thrombin recognition site) at the 5' end.
[0136] The pET28aWaVP8 plasmid was transformed into the competent cell E.coli TOP10, and the clone was screened for pET28aWaVP8-positive colonies in an LB culture dish with 50 μg / ml of Kanamycin. Inoculate in LB culture medium for amplification, save the expression strain below -20°C, and extract the pET28aWaVP8 plasmid f...
Embodiment 3
[0137] Embodiment 3 expression recombinant VP8 protein
[0138] 1. Transform the pET28aWaVP8 plasmid prepared in Example 2 into BL21(DE3) competent cells, inoculate the cells into 50 μg / ml kanamycin LB culture dishes, and inoculate at 37°C in CO 2 overnight in the incubator.
[0139] 2. Pick out a colony, inoculate it into 10 milliliters of kanamycin LB medium (1% tryptone, 0.5% yeast extract, 1% NaCl, pH 7.5) containing 50 μg / ml for amplification, and culture at 37°C overnight.
[0140] 3. Transfer the culture solution to 100 ml of 50 μg / ml kanamycin LB culture solution and continue to cultivate. When the OD of absorbance at 600nm reaches 1.0, inoculate the culture solution into 6 liters to 50μg / ml kanamycin LB culture solution, and continue culturing in a shaker at 37°C with a shaking speed of 200rpm until the OD of absorbance at 600nm When it reaches 0.6-0.8, add 0.3mM IPTG (isopropyl-β-D-thiogalactoside) to induce the expression of VP8.
[0141] 4. Under the same cultu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com