Conjugate vaccines for the prevention of dental caries
a technology of conjugating vaccines and caries, which is applied in the direction of antibody medical ingredients, transferases, immunological disorders, etc., can solve the problems of tooth loss, the possibility of controlling caries by active immunization is currently under intensive investigation, and none of these vaccines has, by itself, proved., and achieves the effect of enhancing the antigenicity of glucan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Glucan Conjugates
Preparation of GTFs:
[0079] GTFs may be purified as described in Smith et al., Infect. Immun., 55:2562-69 (1987); Smith et al., Infect. Immun. 61:2899-2905 (1993); Taubman et al., J. Oral. Pathol. 17:466-70 (1988); and Taubman et al., Infect. Immun. 63:3088-93 (1995), incorporated by reference. Briefly, S. sobrinus 6715 or S. mutans SJ32 are grown in glucose-containing defined media, GTFs, generally comprising GTF-I, GTF-U, and GTF-S, are isolated from culture media by affinity chromatography on Sephadex G-100 (Pharmacia), with 3 M guanidine HCl as the eluting solvent. GTF-rich eluate is applied to fast-performance liquid chromatography on Superose 6 (Pharmacia) with 3 M guanidine HCl for Elution.
Preparation of Streptococcal Mutans Glucans:
[0080] WIG and WSG are prepared as follows: S. mutans, or S. sobrinis 6715 are grown overnight in defined medium containing sucrose, then centrifuged to remove bacterial cells. The cell-free medium containing G...
example 2
Immunogenicity of Streptococcal Mutans Glucans
[0114] Groups of 3 Sprague-Dawley rats were immunized with 1 or 10 μg of WIG, or WSG, or PBS, each incorporated in Freund's adjuvant (DIFCO). Animals were inoculated by subcutaneous injection in the vicinity of the the salivary gland and lymph nodes on day 0 in complete adjuvant, and on day 14 in incomplete adjuvant. Serum and saliva samples were extracted on d14 and d29 and tested for IgG and IgA reactivity with S. sobrinus glucan.
[0115] The Sprague-Dawley rats used herein are derived from germ-free rats that had been reared in the Area 051 isolator facility of Charles River Laboratories and been found to be free of indigenous mutans streptococci. These rats served as the foundation breeding stock for the dams used in these experiments and are regularly monitored for the absence of mutans streptococci. The mutans-free progeny of the dams are weaned at approximately 21 days and are subsequently fed high-sucrose diet 2000. Taubman and S...
example 3
Enhanced Immunogenicity of Glucan Conjugate Vaccines
[0118] Gnotobiotic Sprague-Dawley rats were immunized subcutaneously in the salivary gland vicinity with PBS, WIG, WSG, Tt, or the Tt conjugates described in Example 1. All polysaccharide inocula were used at doses of 1 or 10 μg (PS Dose). As controls, 1 or 10 μg of tetanus toxoid was injected into Tt animals as controls. Rats were immunized on day 0 with antigen in complete Freund's adjuvant (CFA) and boosted on d14 with the same dose of antigen suspended in incomplete Freund's adjuvant (IFA). Saliva and blood taken from tail veins are collected on d28 and d42 and analyzed for levels of IgG (blood) and IgA (saliva) reactive with WIG, WSG, and Tt.
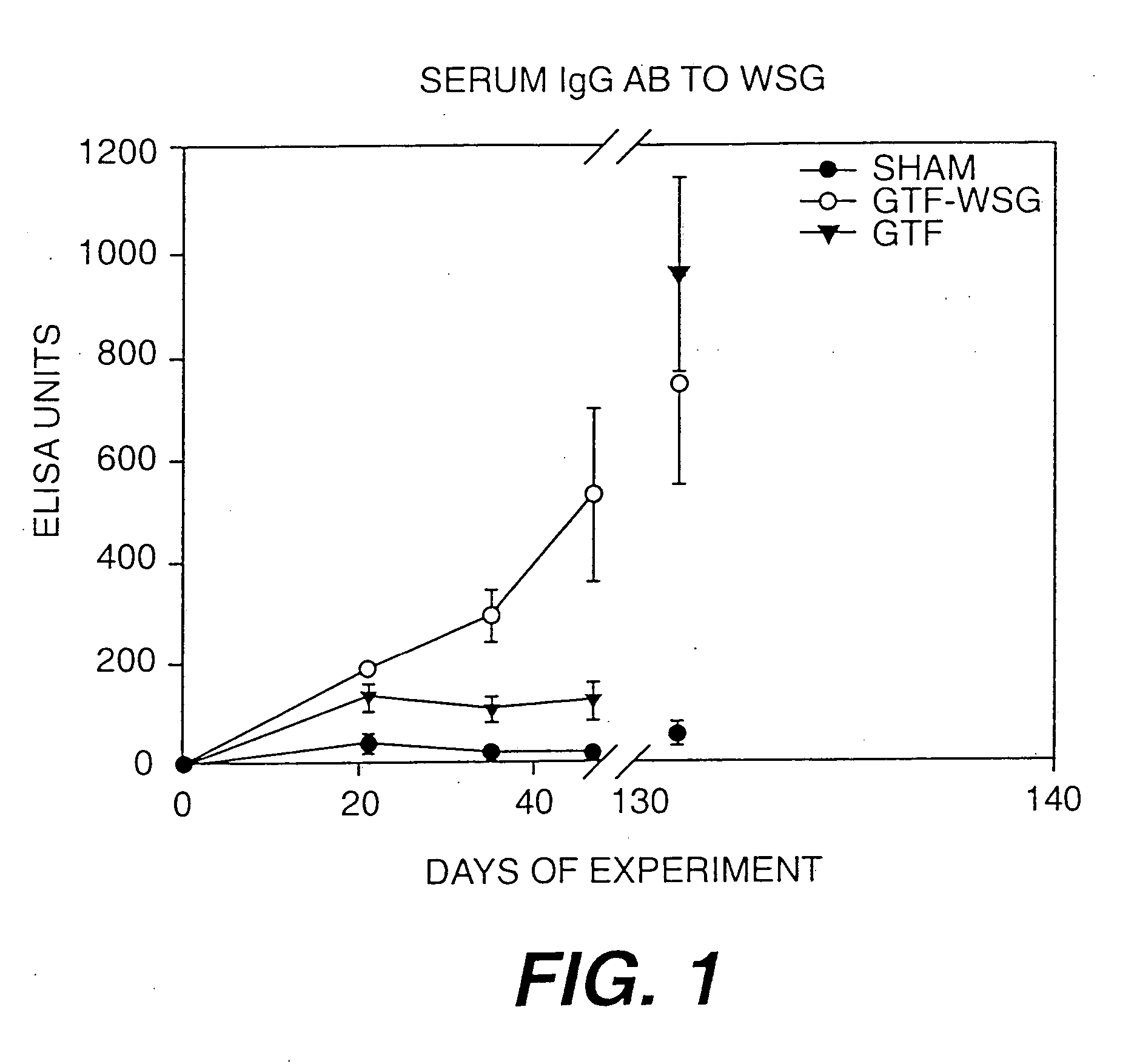
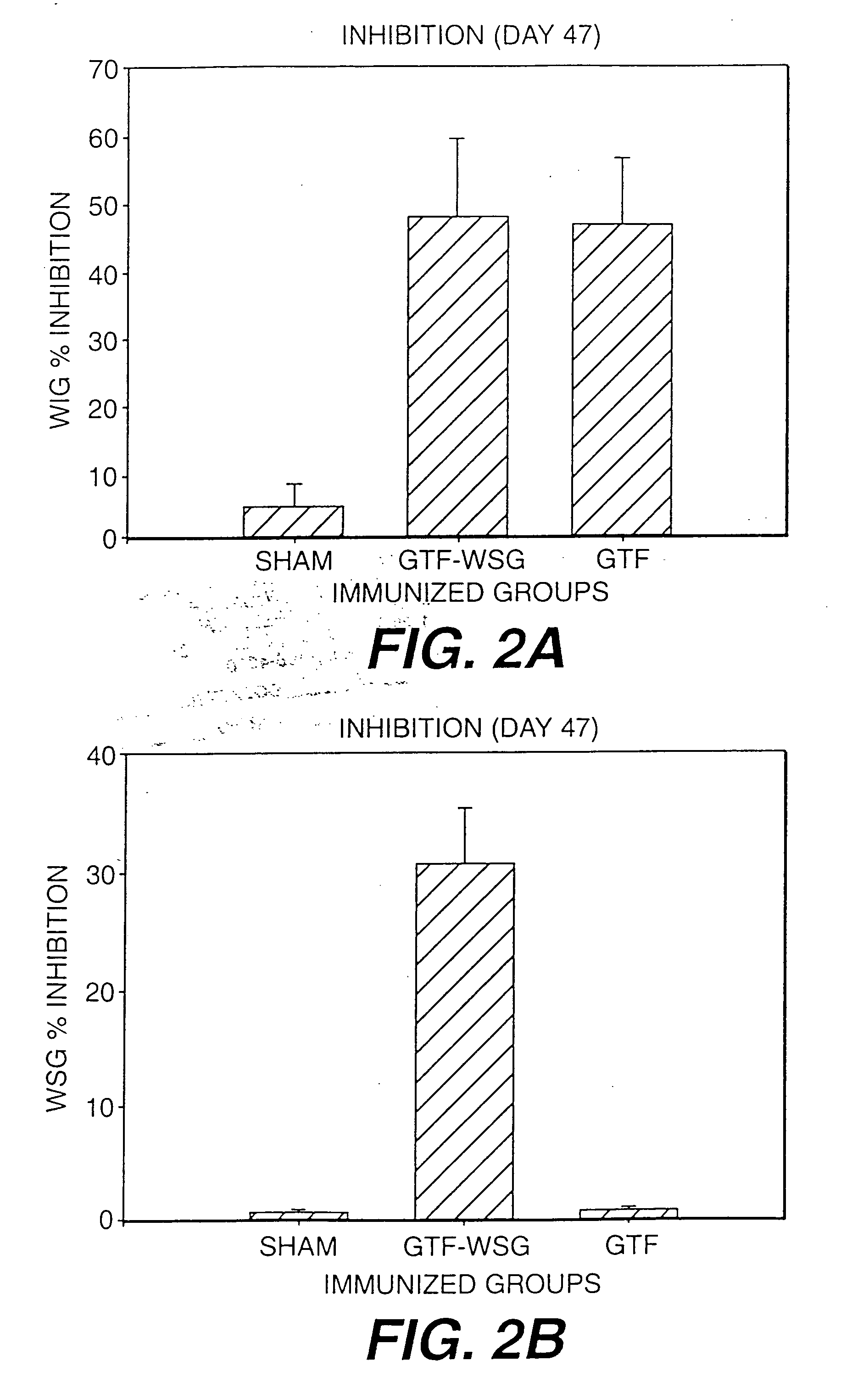
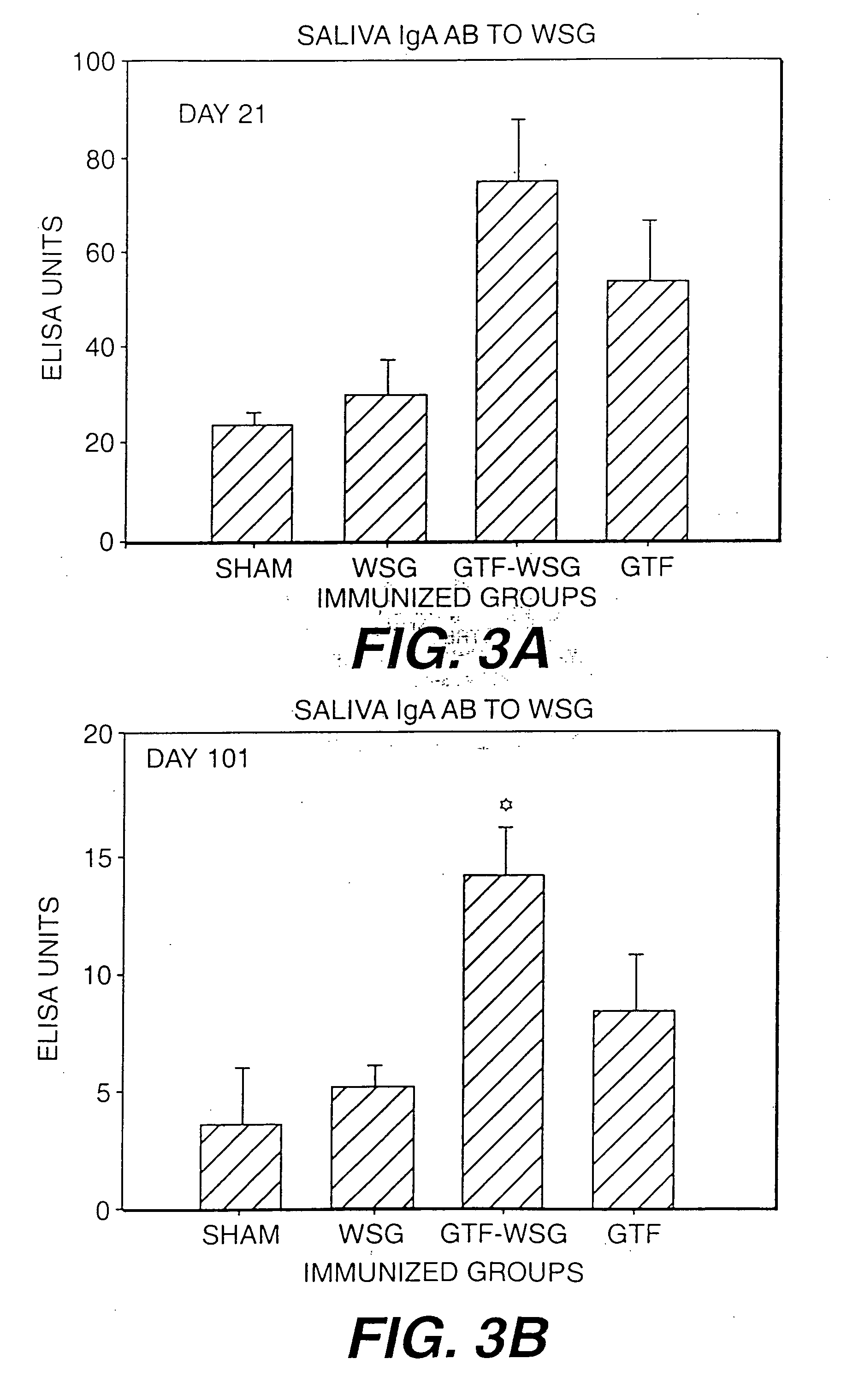
[0119] The compilation of the serum antibody titer data presented in Table I. The results of additional experiments using WSG-GTF conjugates is presented in FIG. 1. In addition, WSG-Tt and WSG-GTF conjugates additionally comprising mutans-derived peptide moities will be prepared.
T cell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com