Preparation method and application of paclitaxel albumin nanoparticles
A technology of albumin nanoparticles and paclitaxel, which is applied in the field of biomedical preparations, can solve the problems of consumption of yew trees, limited extraction of paclitaxel, low solubility of paclitaxel, etc., and achieves improved drug solubility, reduced toxicity or immune response, organic Reagent volume without residue effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
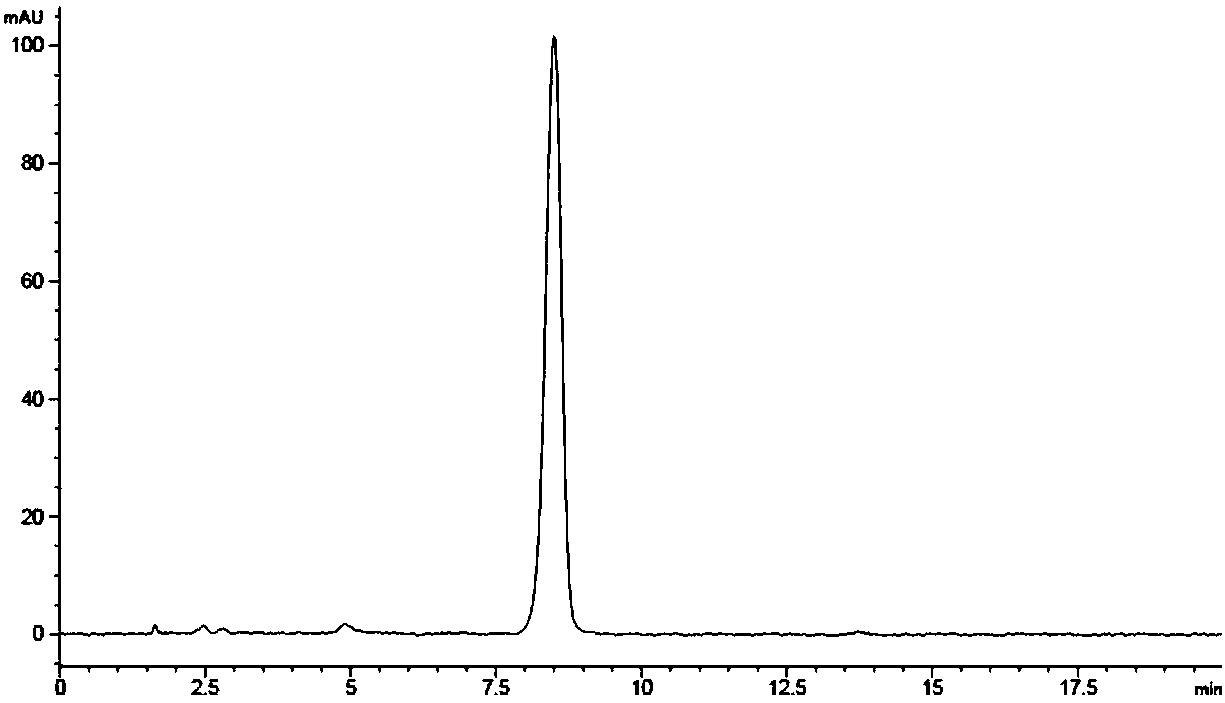
Image
Examples
preparation example Construction
[0034] A preparation method of paclitaxel albumin nanoparticles, comprising the steps of:
[0035] 1) Weigh 50 mg of paclitaxel raw material, dissolve it in 1 mL of organic solvent, and sonicate for 30 min to obtain an oil phase. The organic solvent is dichloromethane, or acetone, or a mixture of dichloromethane and acetone (v:v=1:1).
[0036] 2) Weigh bovine serum albumin and dissolve it in water, stir and heat to make it fully dissolved to obtain a bovine serum albumin aqueous solution with a mass volume percentage of 5% (m / v), and add 1mol / L hydrochloric acid dropwise to adjust the albumin aqueous solution pH to 6.0, adding 1% organic solvent to the total volume of the bovine serum albumin aqueous solution, pre-saturation, to obtain the aqueous phase. The organic solvent is dichloromethane, or acetone, or a mixture of dichloromethane and acetone (v:v=1:1).
[0037] 3) Under the action of high-speed shear (rotating speed 10000r), add the oil phase to the water phase drop b...
Embodiment 1
[0040] (1) Orthogonal experiment design
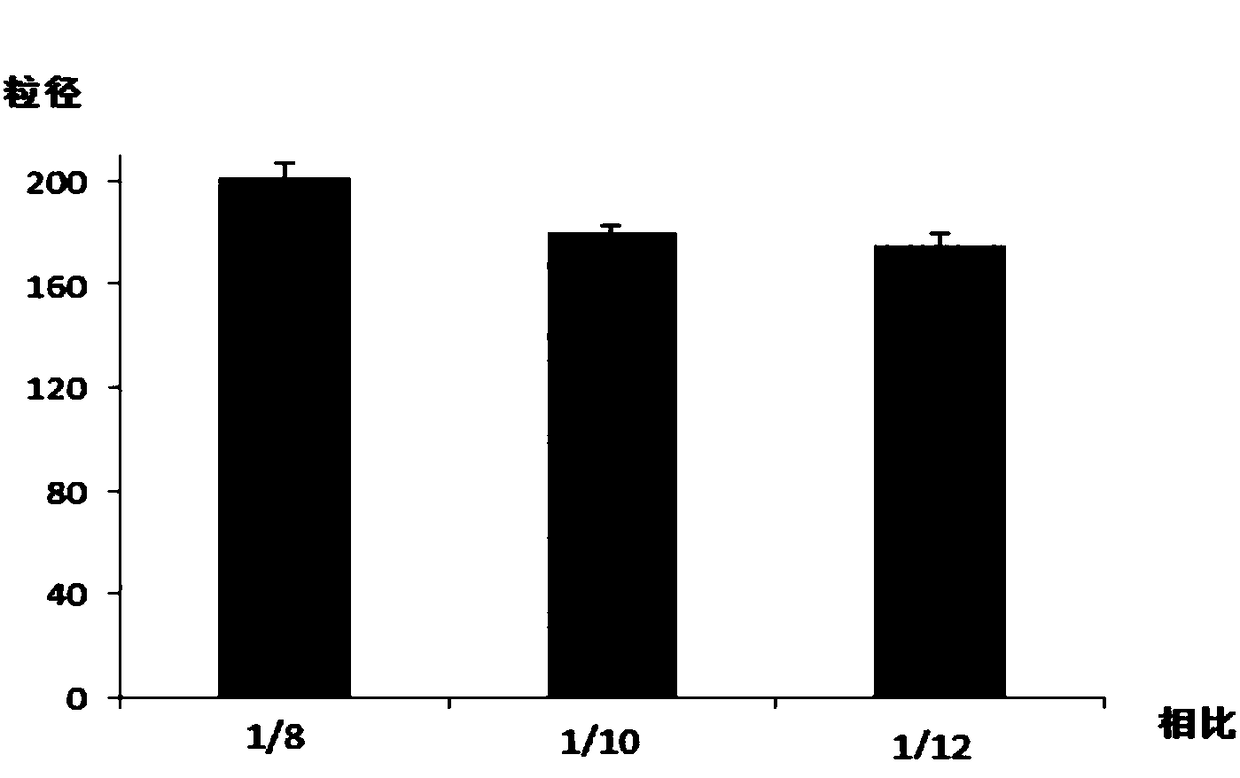
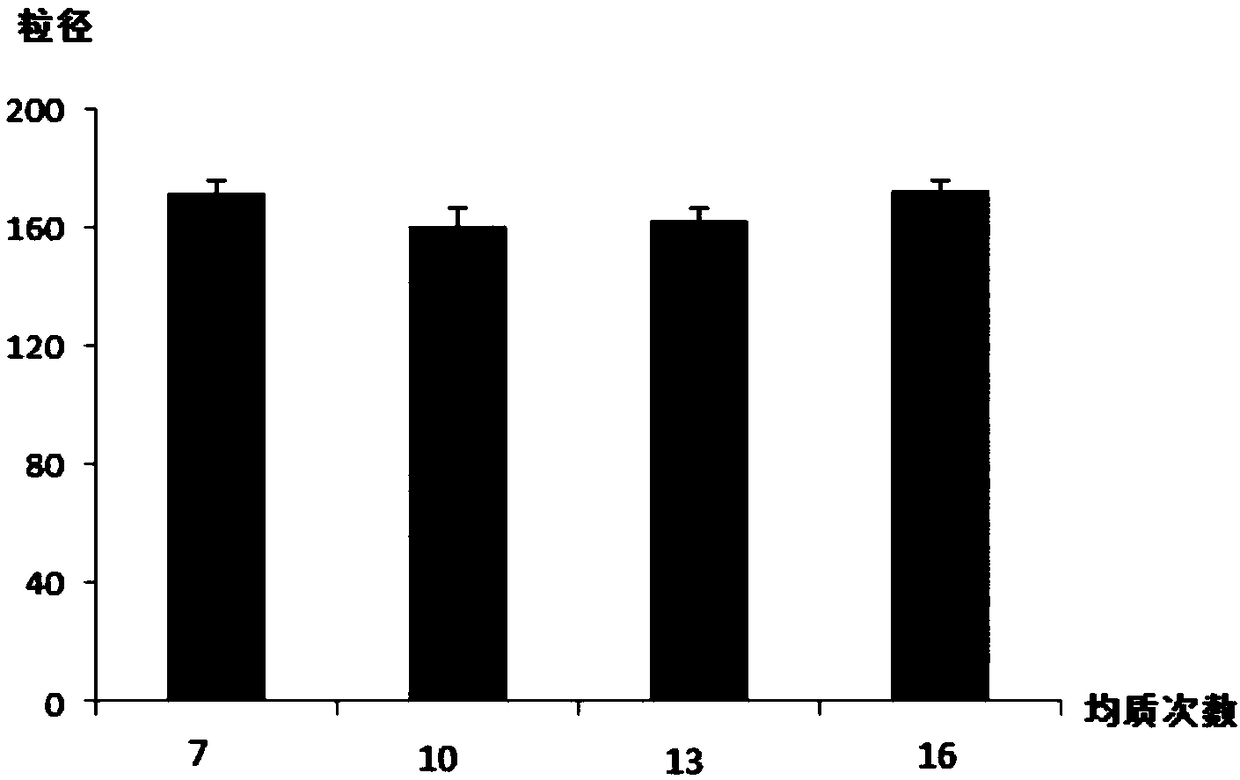
[0041] It can be known from experiments that the type of organic solvent (A), the volume ratio of the oil phase to the water phase (B), and the number of homogenizations (C) are the key factors affecting the particle size of the nanoparticles.
[0042] Type A of organic solvent: 1. Dichloromethane; 2. Acetone; 3. Dichloromethane-acetone (v:v=1:1);
[0043] Volume ratio B of oil phase and water phase: 1, 1:4; 2, 1:7; 3, 1:10;
[0044] Homogenization times C: 1, 5 times; 2, 10 times; 3, 15 times, the experimental design is shown in Table 1:
[0045] Table 1 Orthogonal experiment design table
[0046]
[0047]
[0048] Table 2 Orthogonal test results
[0049]
[0050] Orthogonal design analysis form is shown in Table 2, K in the table 1 、K 2 、K 3 R is the average value of the experimental results (particle size) under the three levels of the corresponding factor, and the R value is the range of each factor, that is, the dif...
Embodiment 2
[0063] (1) a preparation method of paclitaxel albumin nanoparticles, comprising the steps of:
[0064] 1) Weigh 50 mg of the paclitaxel raw material, dissolve it in 1 mL of acetone, and sonicate for 30 min to obtain an oil phase.
[0065] 2) Weigh bovine serum albumin and dissolve it in water, stir and heat to make it fully dissolved to obtain a bovine serum albumin aqueous solution with a mass volume percentage of 5% (m / v), and add 1mol / L hydrochloric acid dropwise to adjust the albumin aqueous solution To bring the pH to 6.0, add 1% acetone to the total volume of the bovine serum albumin solution.
[0066] 3) Under the action of high-speed shear (rotating speed 10000r), add the oil phase to the water phase drop by drop to make an oil-in-water emulsion. By volume, oil phase: water phase = 1:10.
[0067] 4) Transfer the oil-in-water emulsion to a high-pressure micro-fluidic nanodisperser for high-pressure homogenization, the homogenization pressure is 1000 bar, and the homog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com