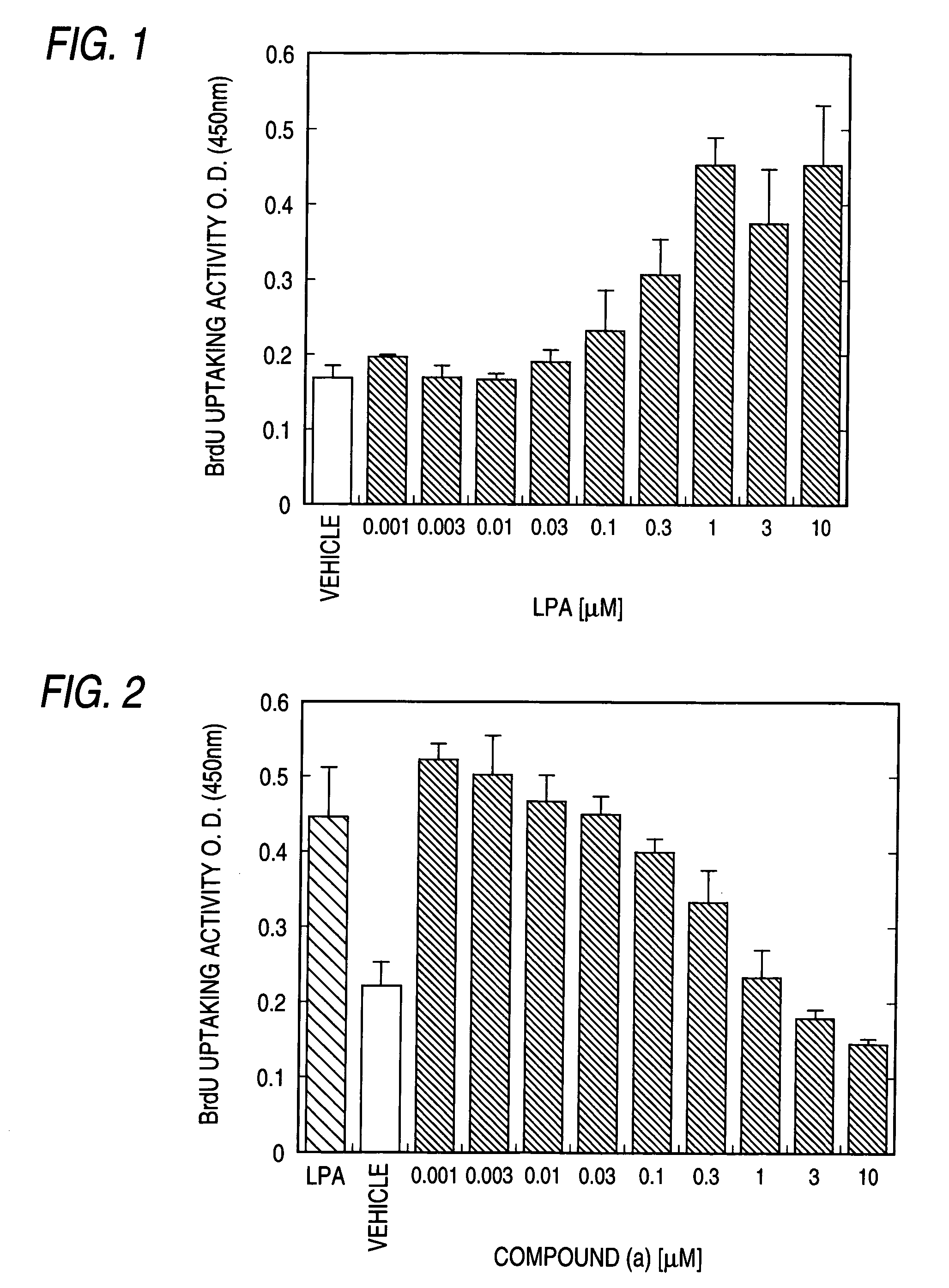
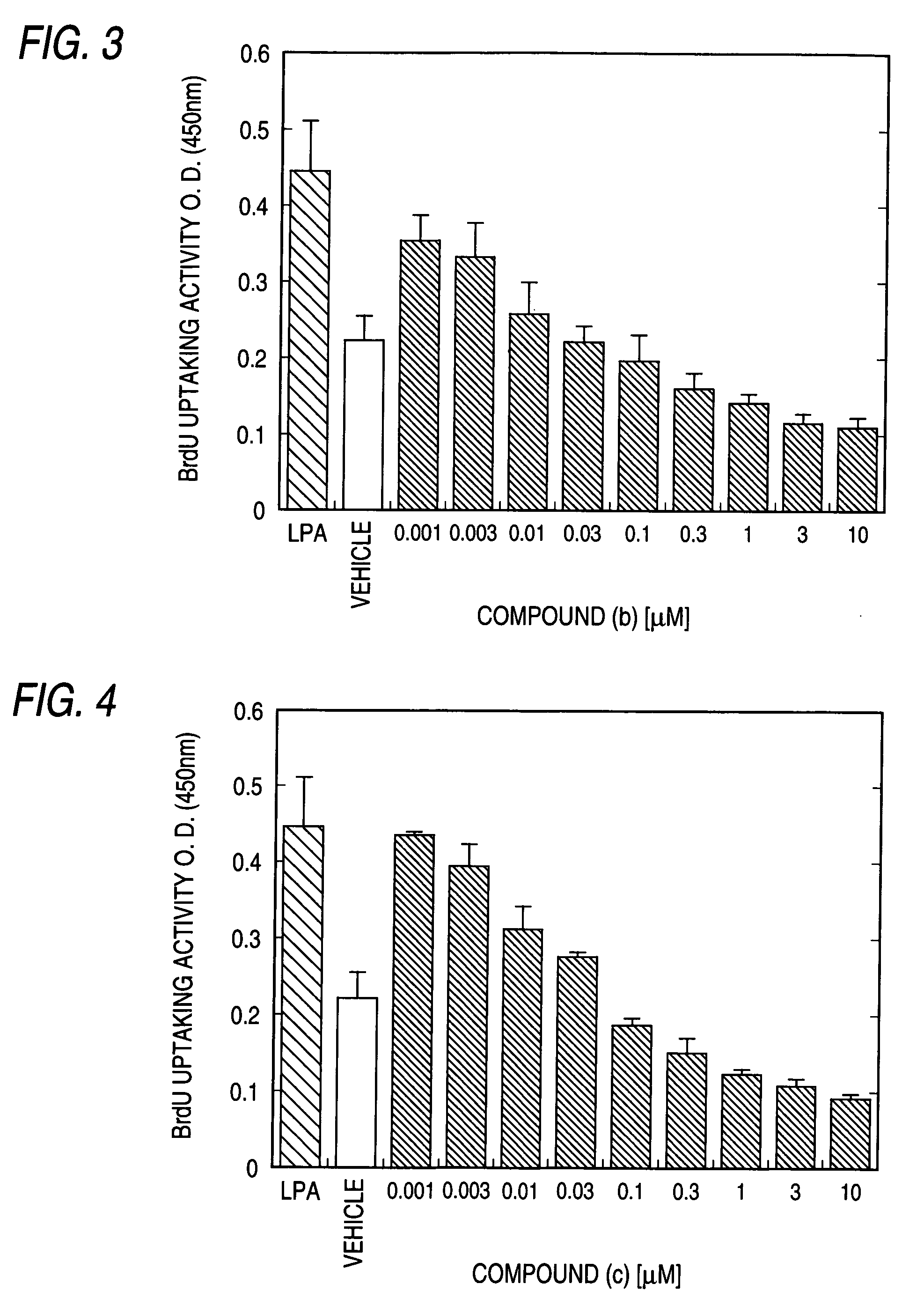
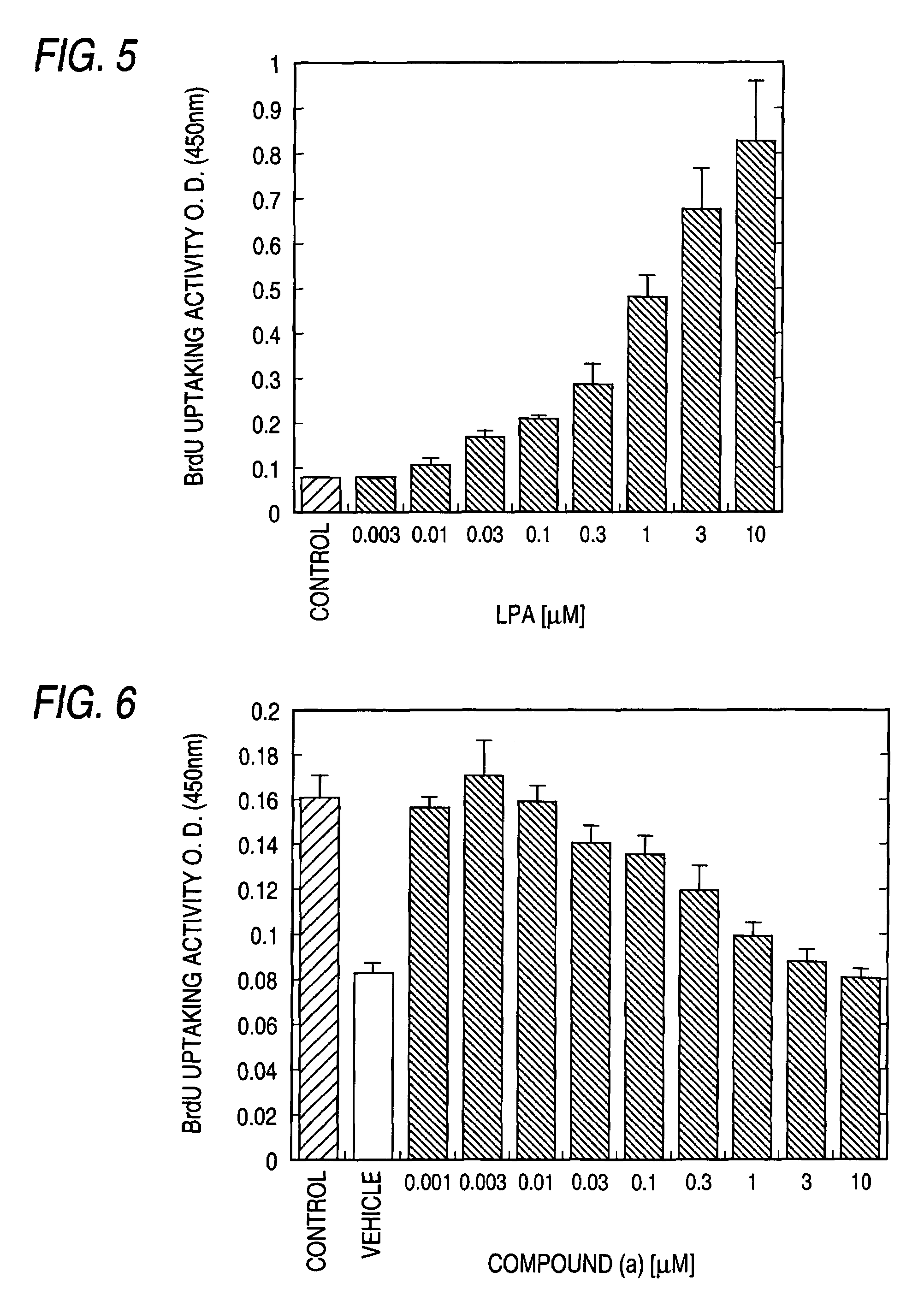
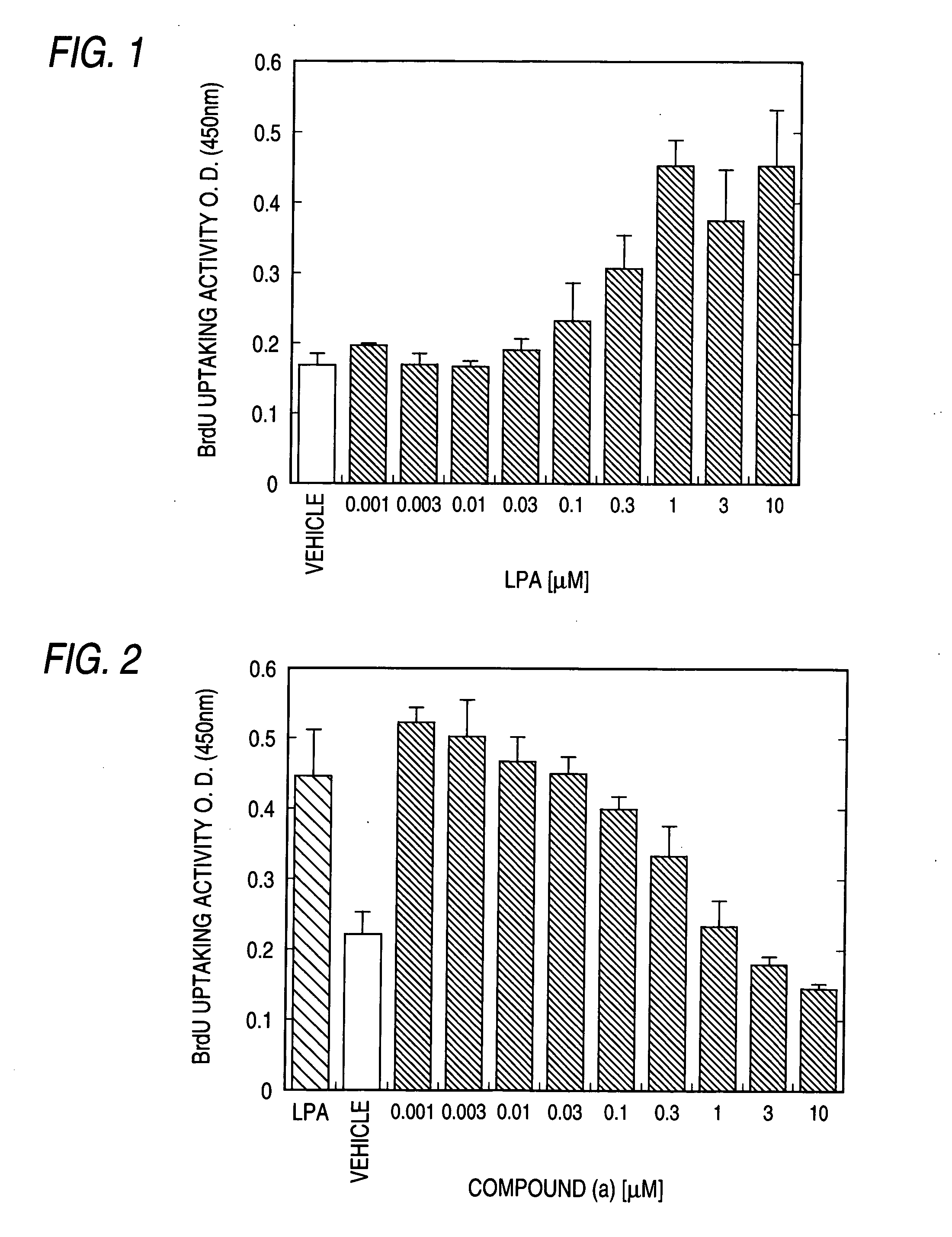
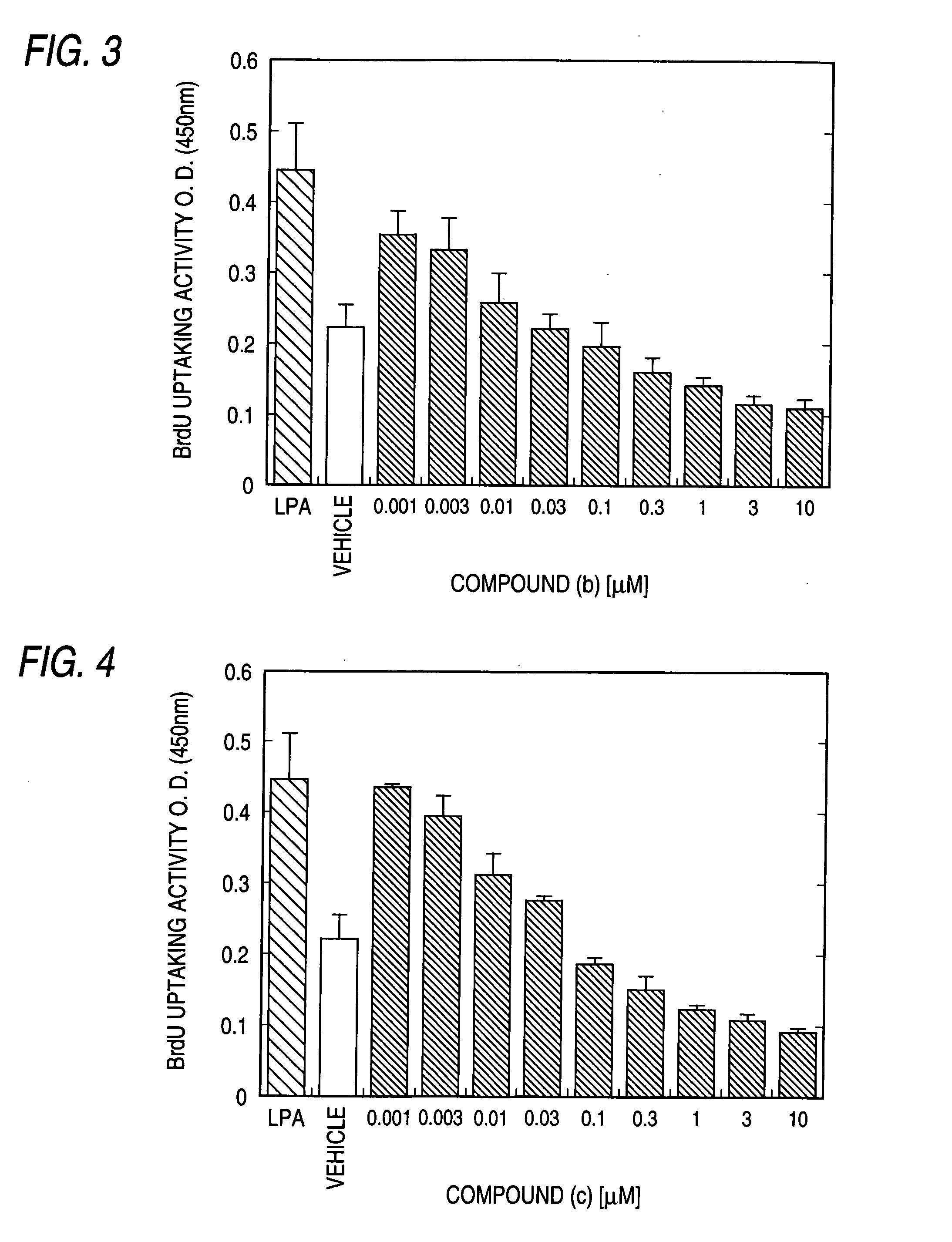
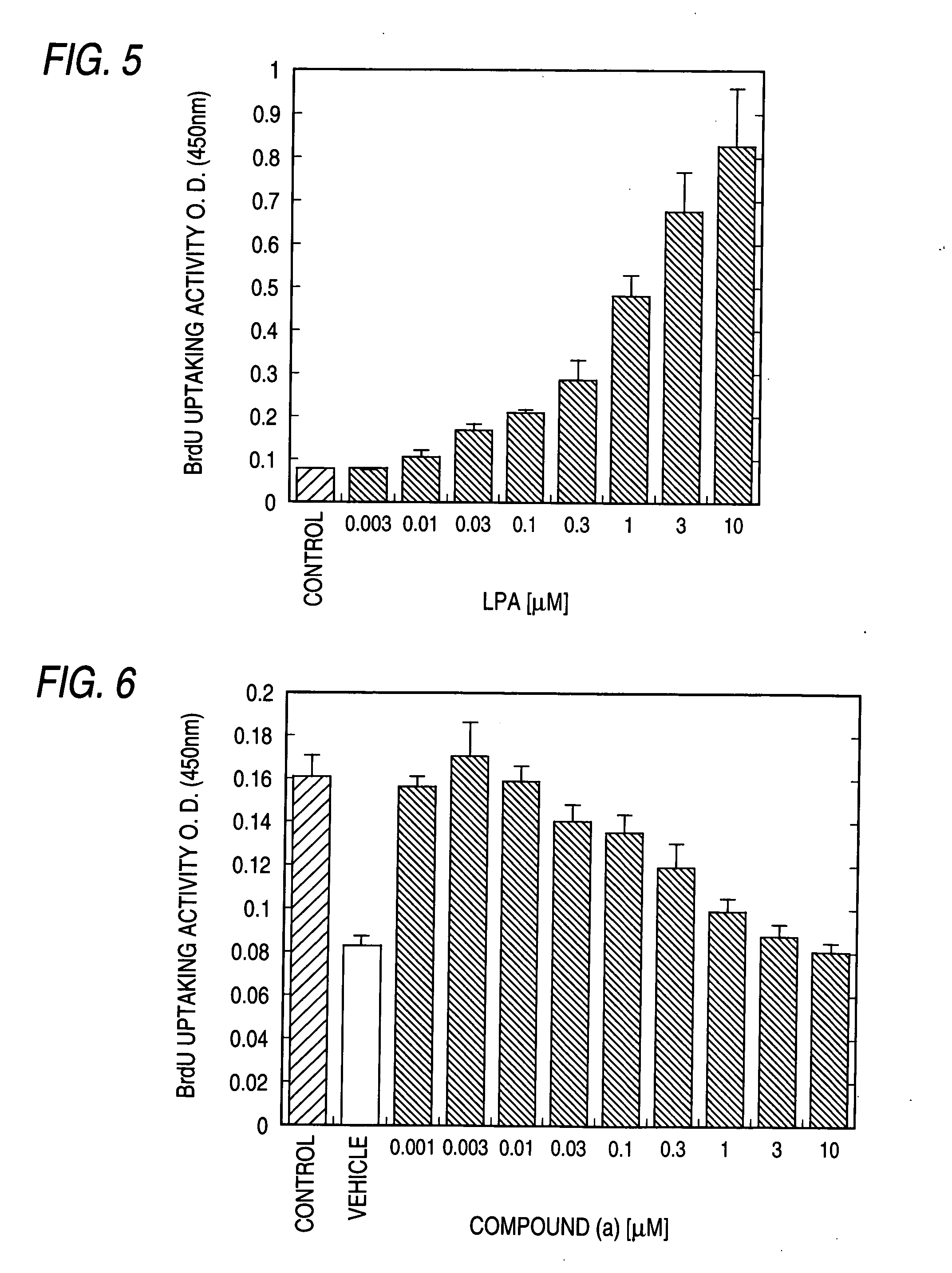
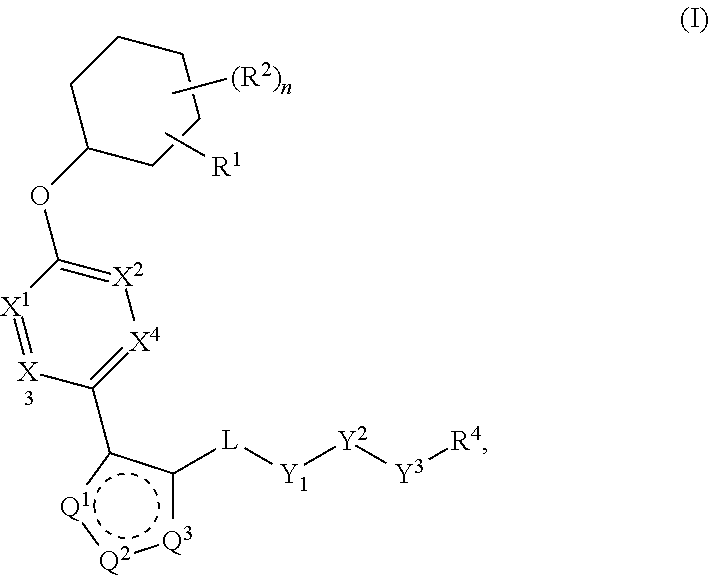
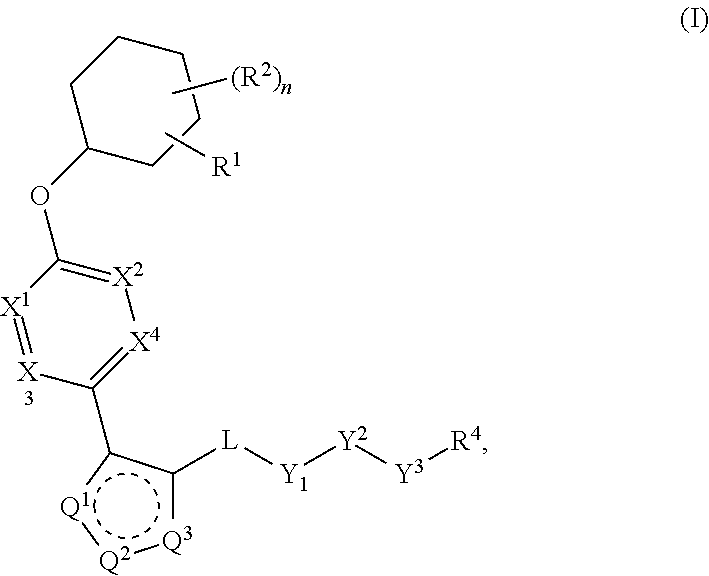
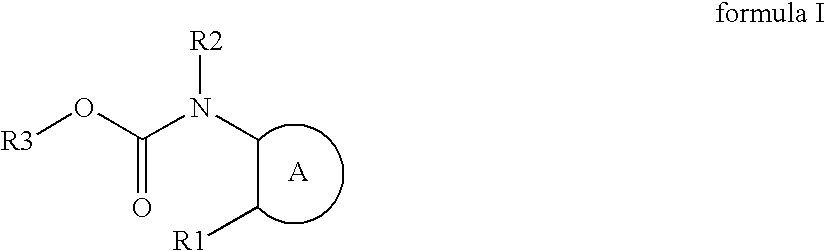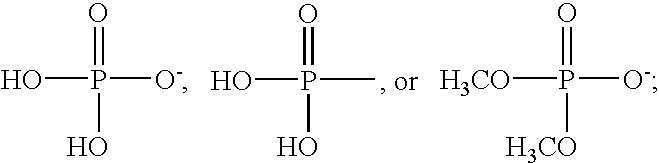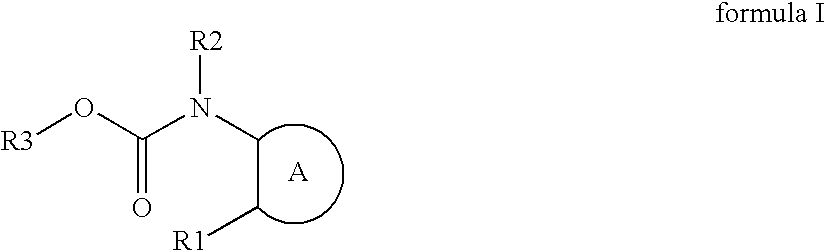Patents
Literature
49 results about "LPA Receptors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
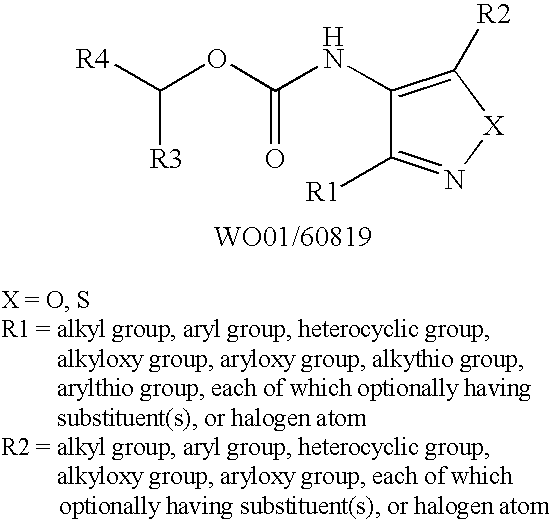
Novel azole compound
InactiveUS20060194850A1Improve liver functionInhibition of physiological activityBiocideNervous disorderDiseaseHydrogen atom
Azole compounds represented by formula I: wherein ring A is isoxazole and the like, R1 is a substituted or unsubstituted aryl group and the like, R2 is a hydrogen atom and the like, and R3 is a substituted or unsubstituted alkyl group and the like, and pharmaceutically acceptable salts thereof inhibit the physiological activity of lysophosphatidic acid (LPA), and are useful as for the prophylaxis or treatment of diseases in which inhibition of the physiological activity of LPA is useful for the prophylaxis or treatment thereof, such as diseases involving the LPA receptor.
Owner:AJINOMOTO CO INC
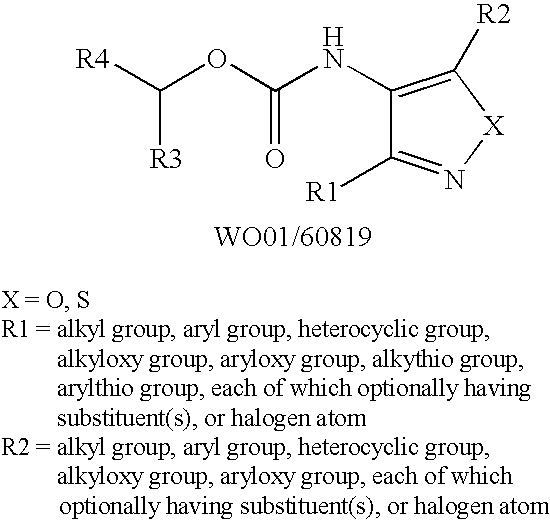
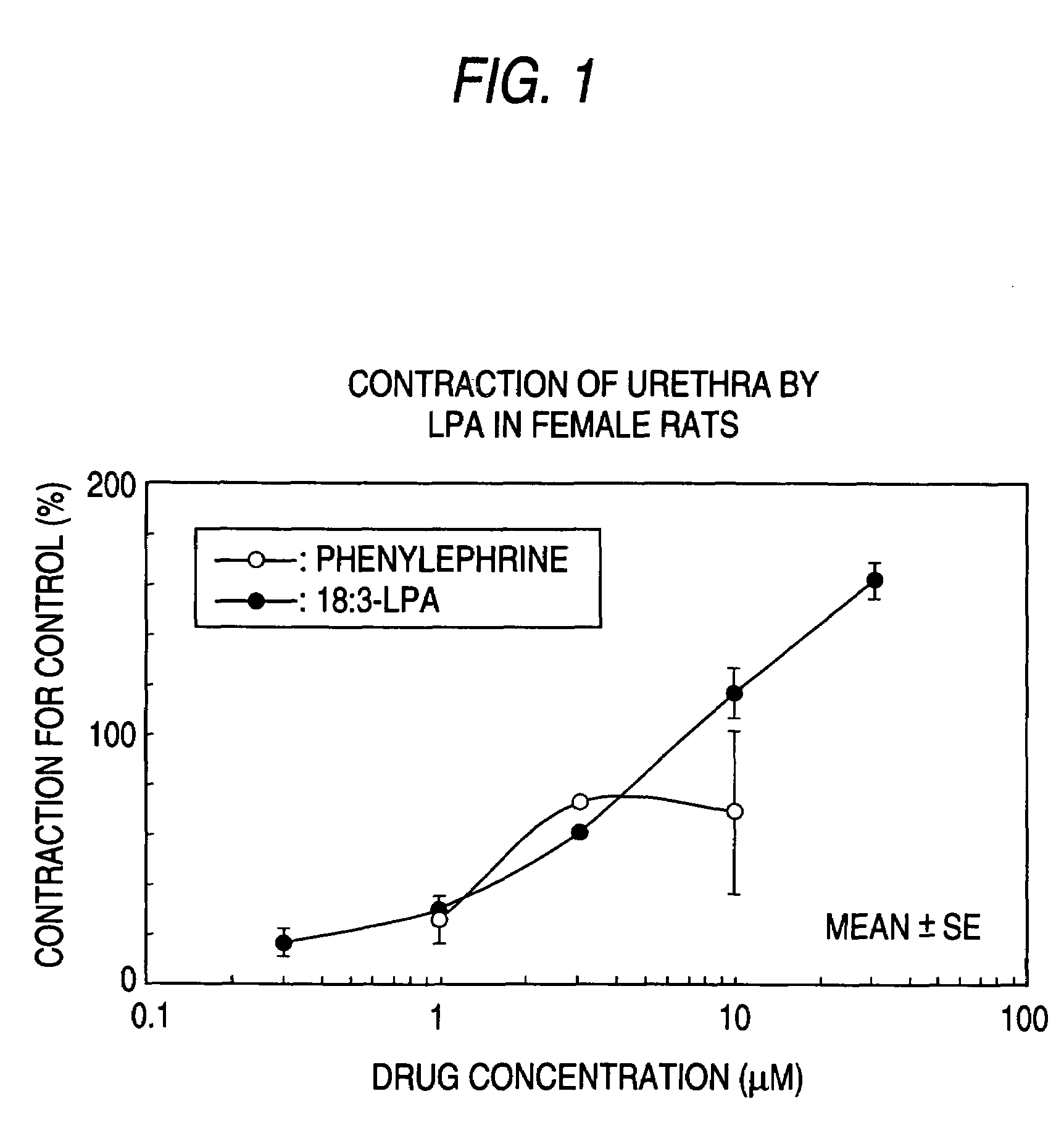
Pharmaceutical composition for treatment for urinary diseases comprising LPA receptor regulator
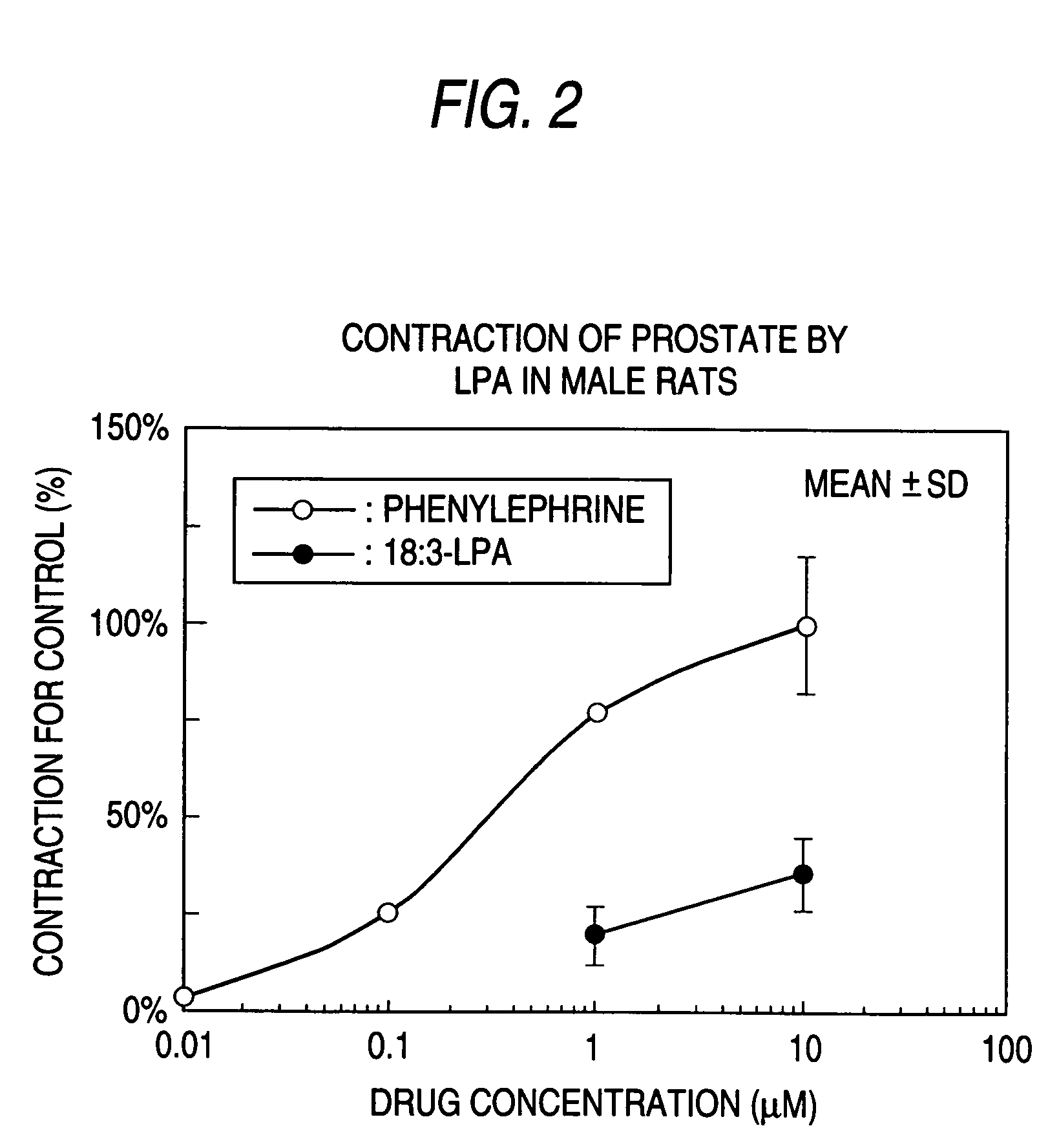
The present invention relates to a pharmaceutical composition for treatment and / or prevention for urinary diseases comprising a lysophosphatidic acid (LPA) receptor regulator.As LPA receptor regulators act on urethra and prostate, compounds acting on this receptor are useful in treating urinary diseases (urinary incontinence, dysuria, etc.). For example, an LPA receptor agonist useful for treatment of urinary incontinence, while an LPA receptor antagonist is useful for treatment of dysuria, ischuria, pollakiuria, nocturia, urodynia and benign prostatic hyperplasia.
Owner:ONO PHARMA CO LTD
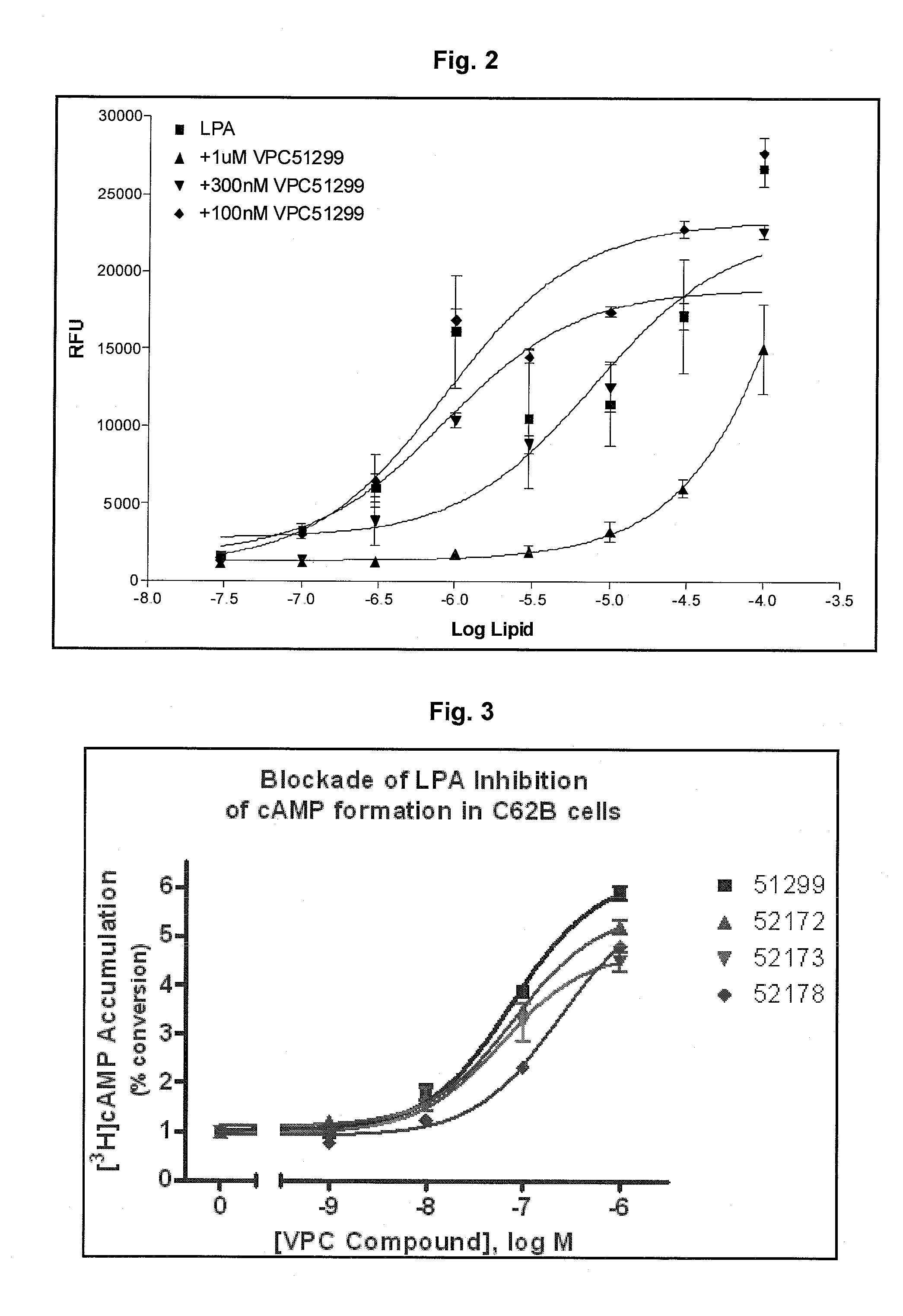
LPA analogs as agonists of the Edg2 LPA receptor
InactiveUS6380177B1Improve bindingBiocidePhosphorous compound active ingredientsDiseaseLPA Receptors
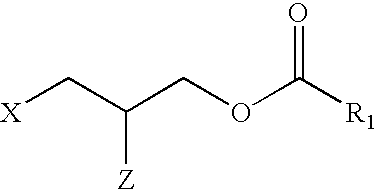
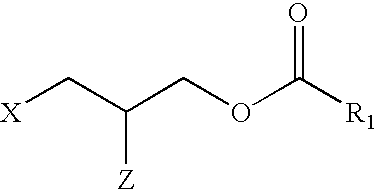
Applicant has probed the Edg2 lysophosphatidic acid (LPA) receptor with a series of LPA analogs to determine receptor activation. The present invention is drawn to a series of LPA analogs which function as Edg2 receptor agonists, and methods of using such compounds to activate the Edg2 receptor of the surface of a cell. The compounds of the invention comprise a glycerol backbone with an Sn1 ester-linked saturated or unsaturated alkyl group, substitutions of the hydroxyl group (-OH) at carbon two of the glycerol backbone, and optional replacement of the phosphate di-anion with either a hydroxyl group or a dimethylated phosphate. These LPA analogs may find uses in cancer and neurological disorders.
Owner:APOLLO ENDOSURGERY INC
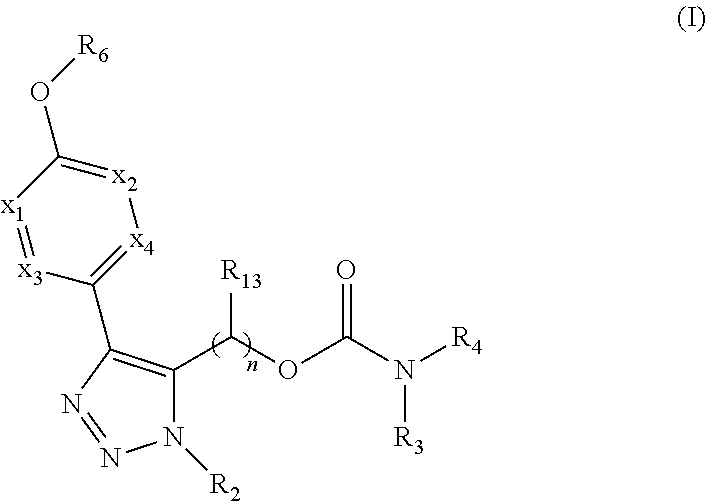
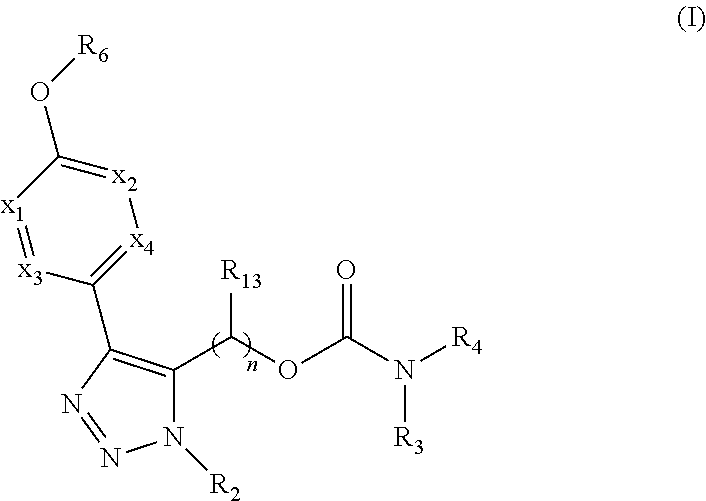
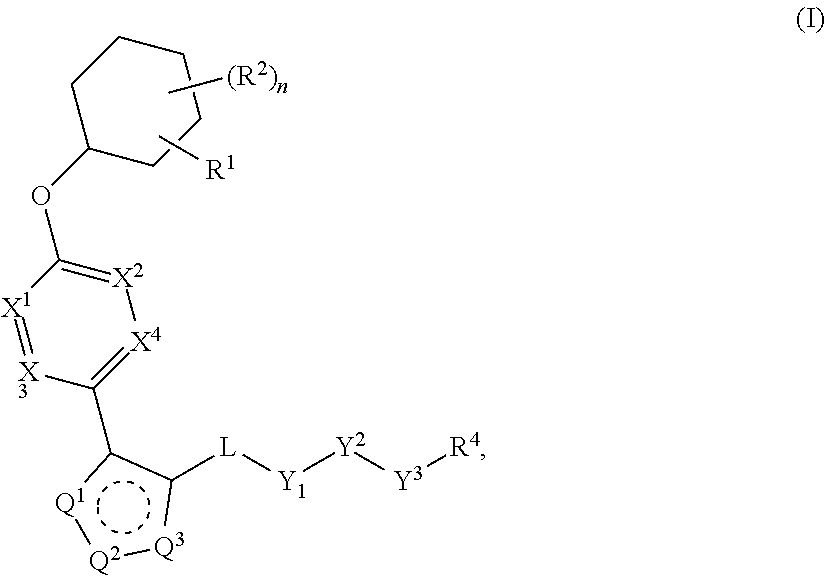
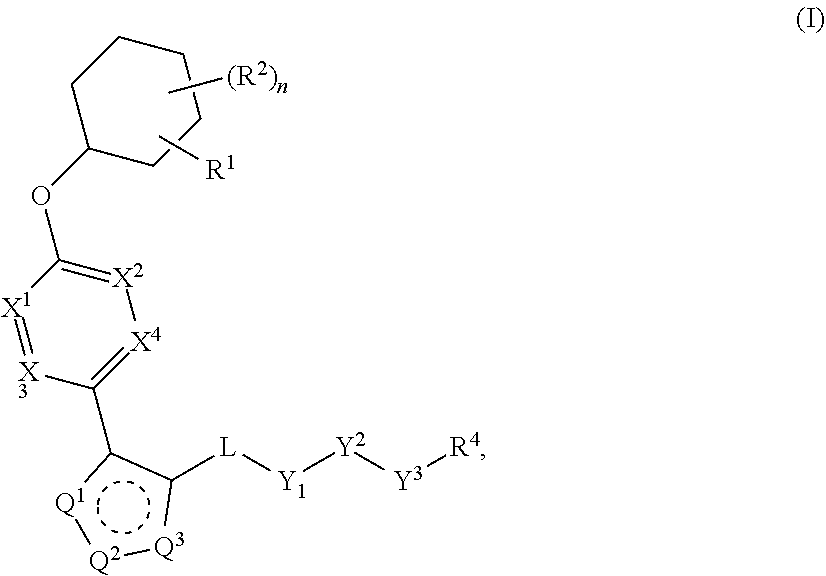
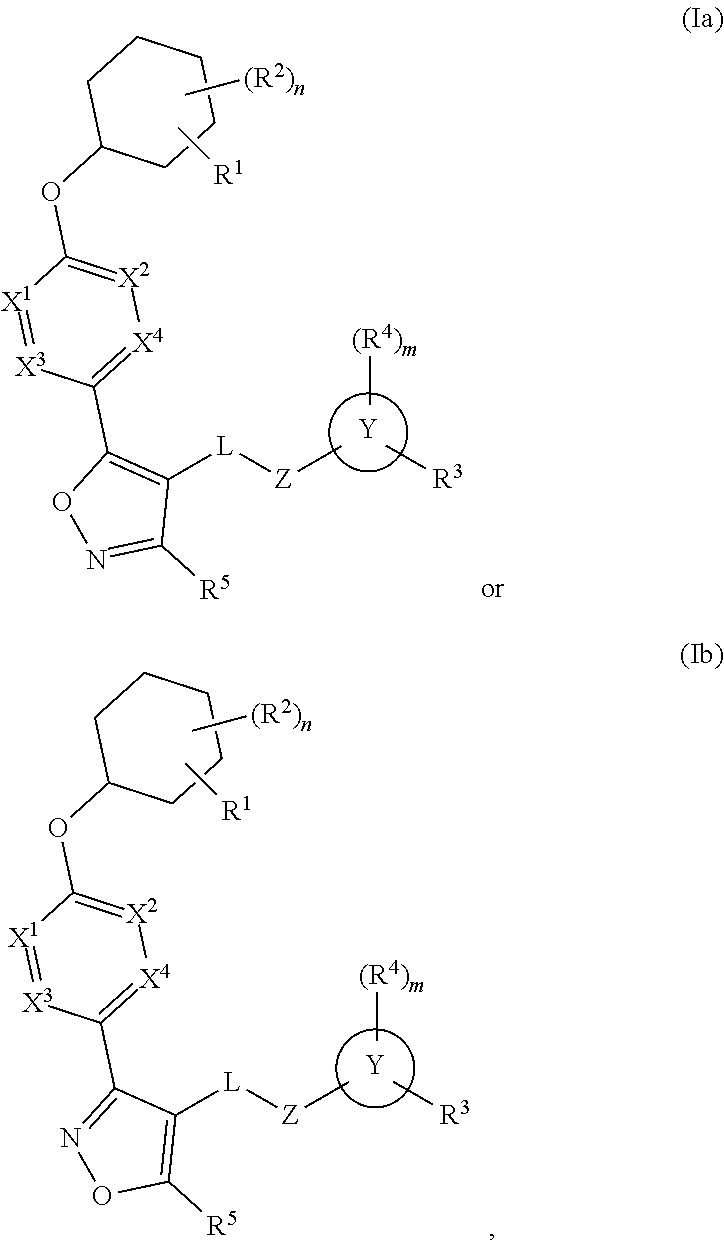
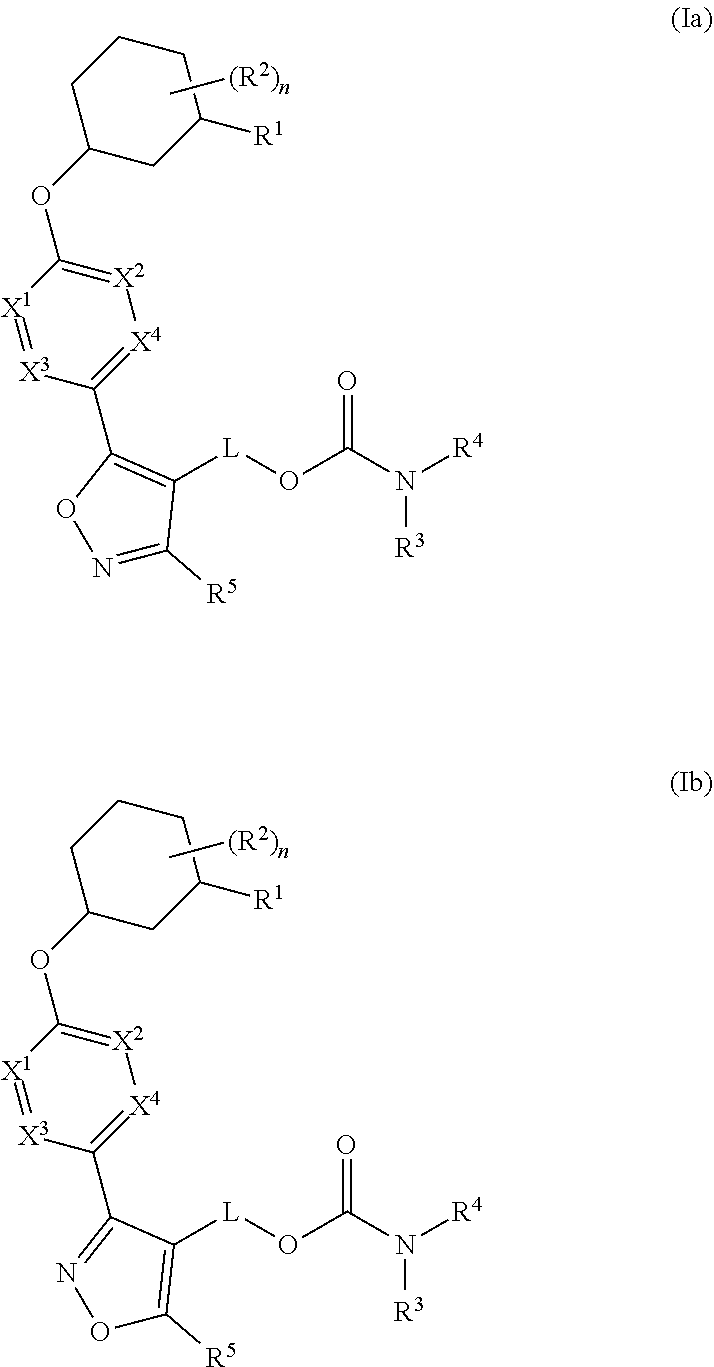
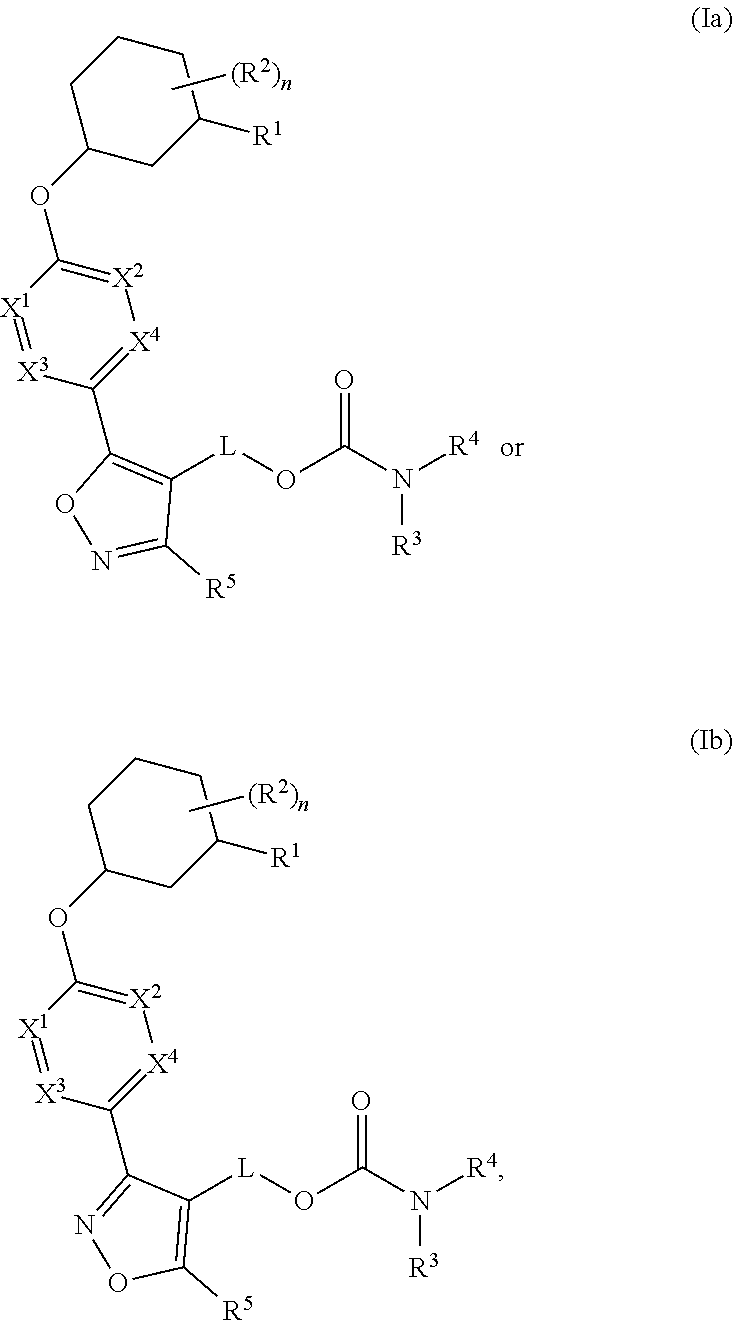
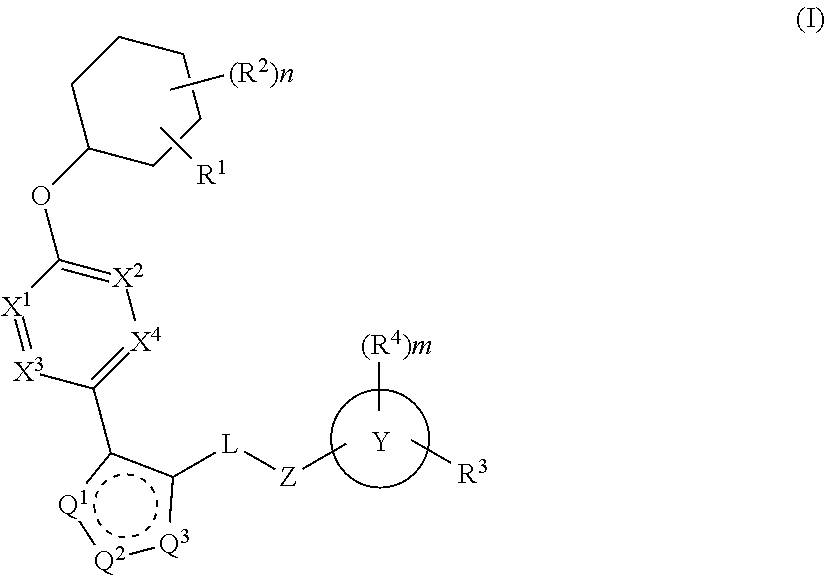
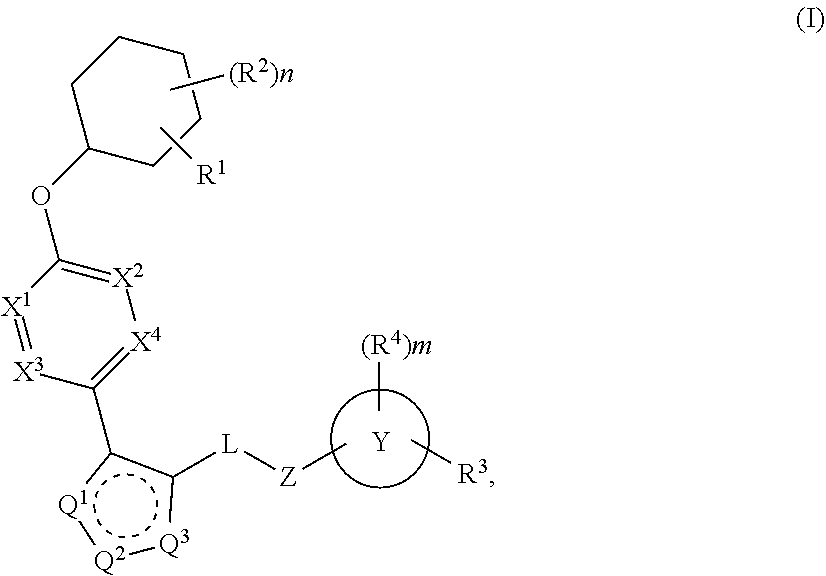
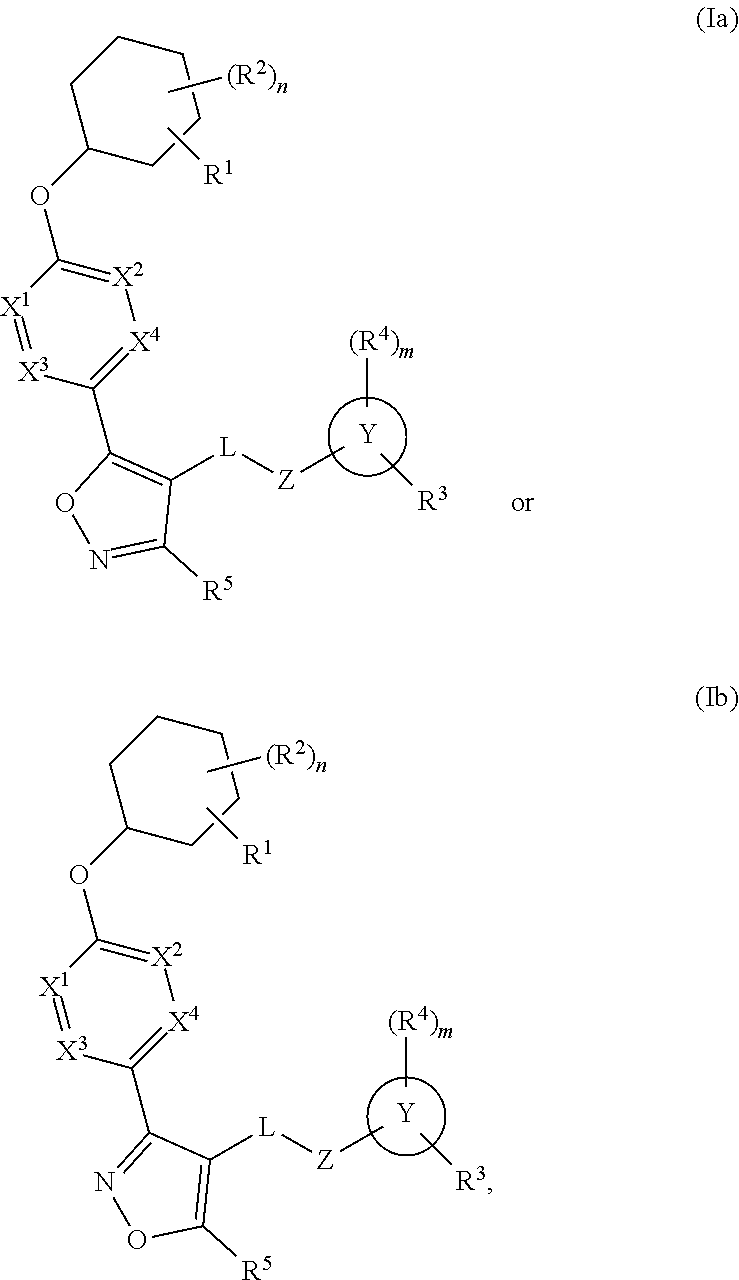
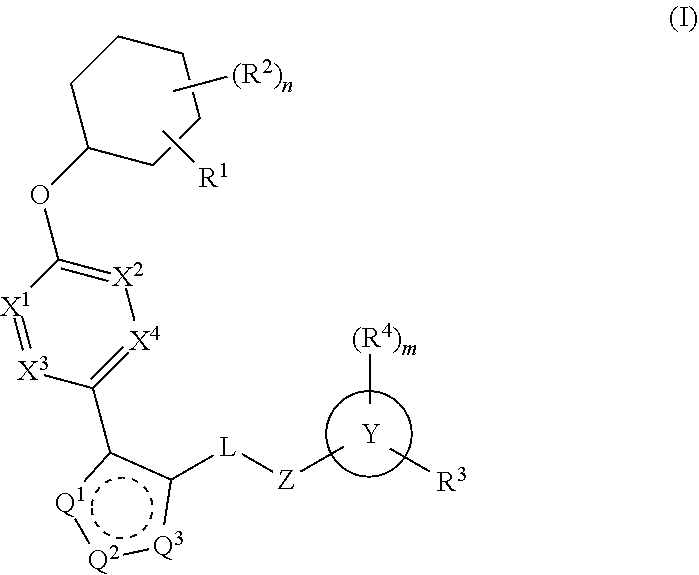
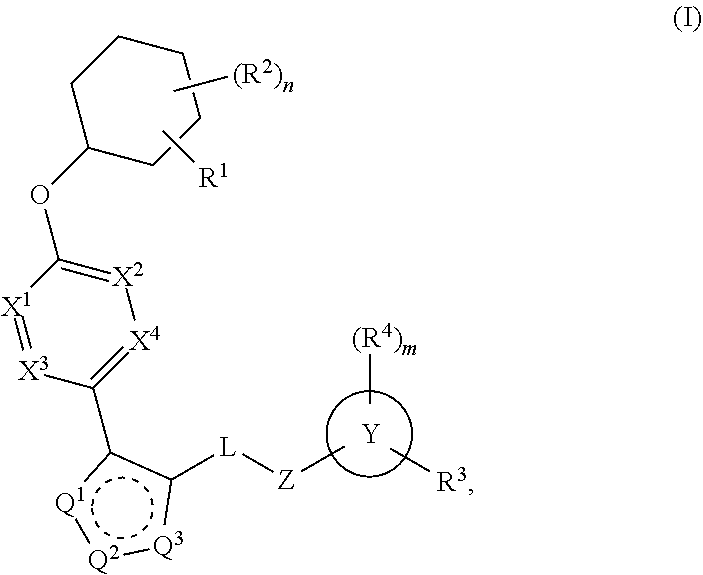
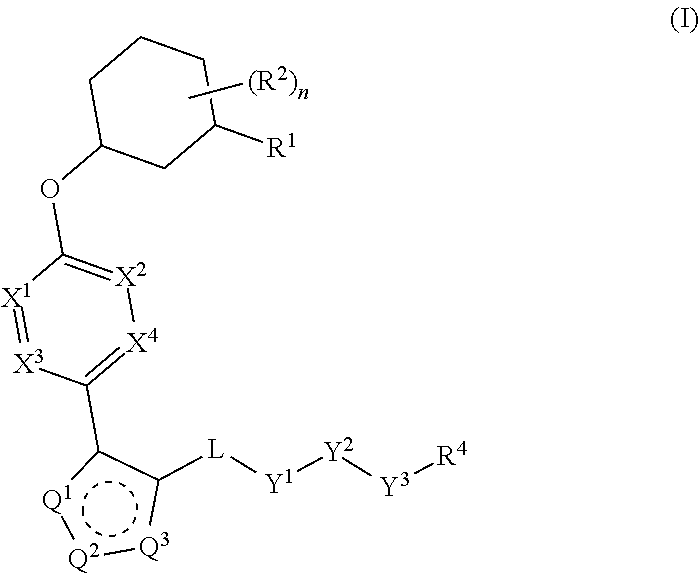
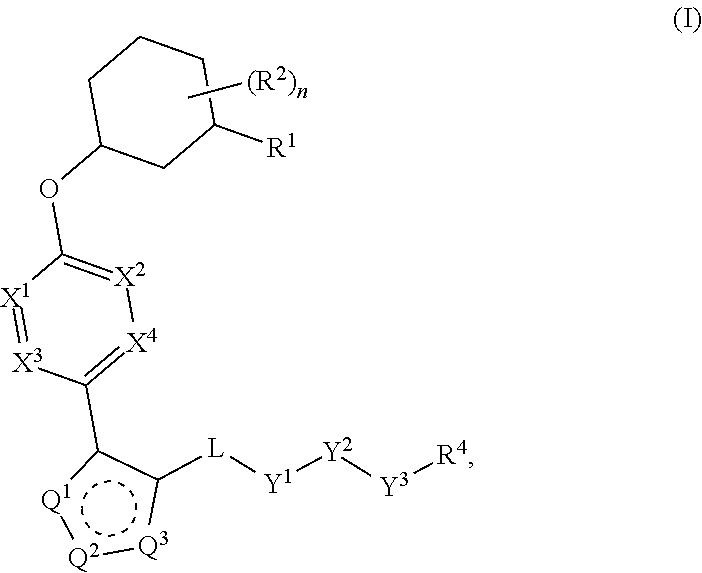
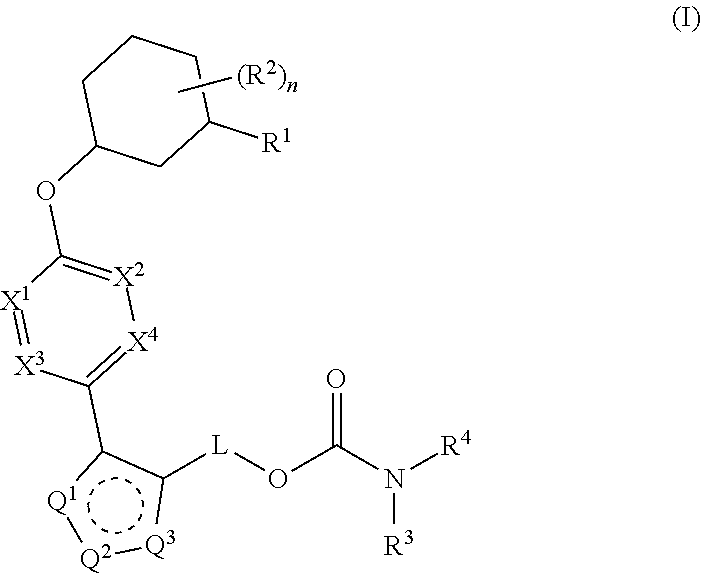
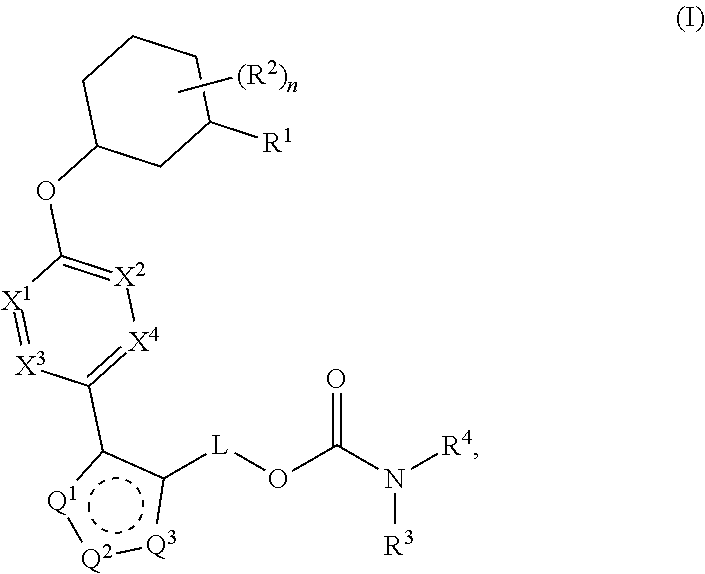
Carbamoyloxymethyl triazole cyclohexyl acids as lpa antagonists
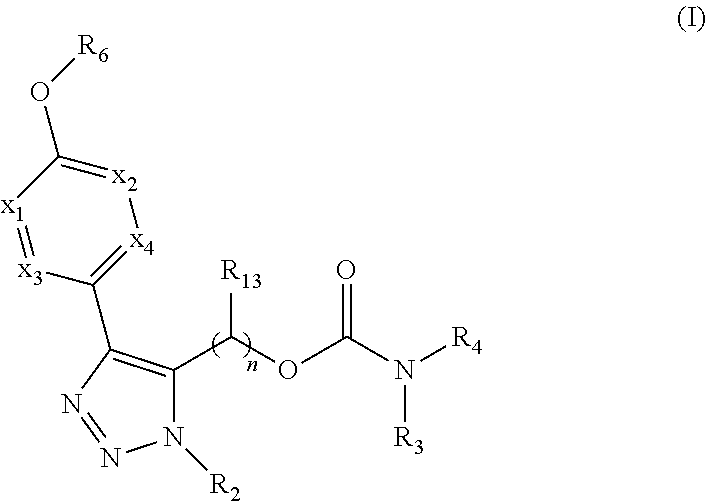
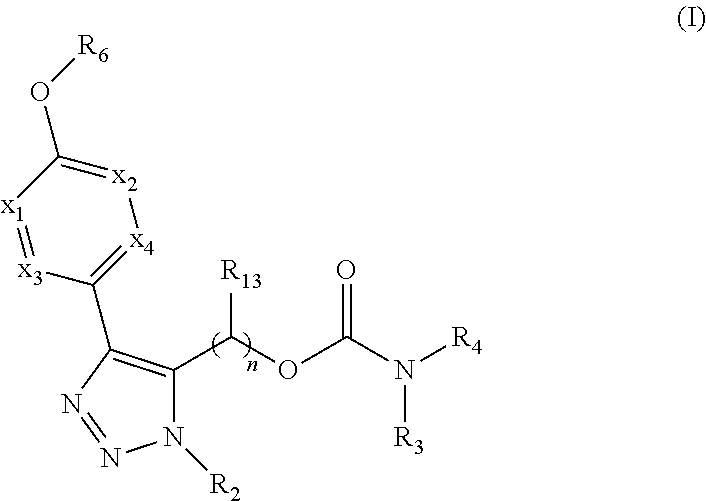
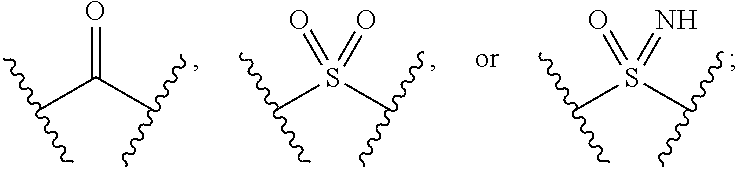
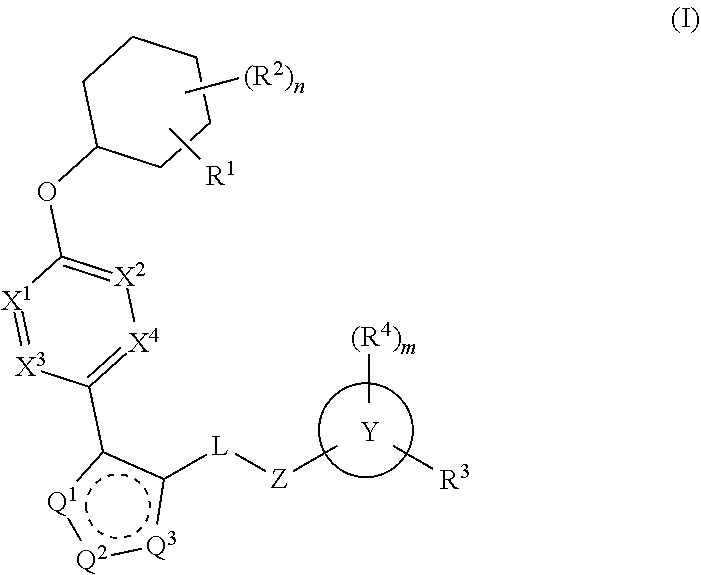
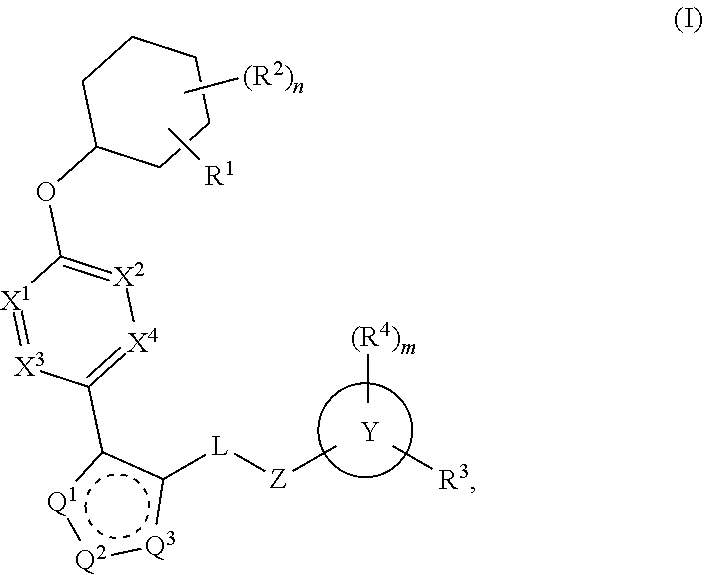
The present invention provides compounds of Formula (I):or stereoisomers, tautomers, pharmaceutically acceptable salts, solvates or prodrugs thereof, wherein all the variables are as defined herein. These compounds are selective LPA receptor inhibitors.
Owner:BRISTOL MYERS SQUIBB CO
Remedy for chronic disease
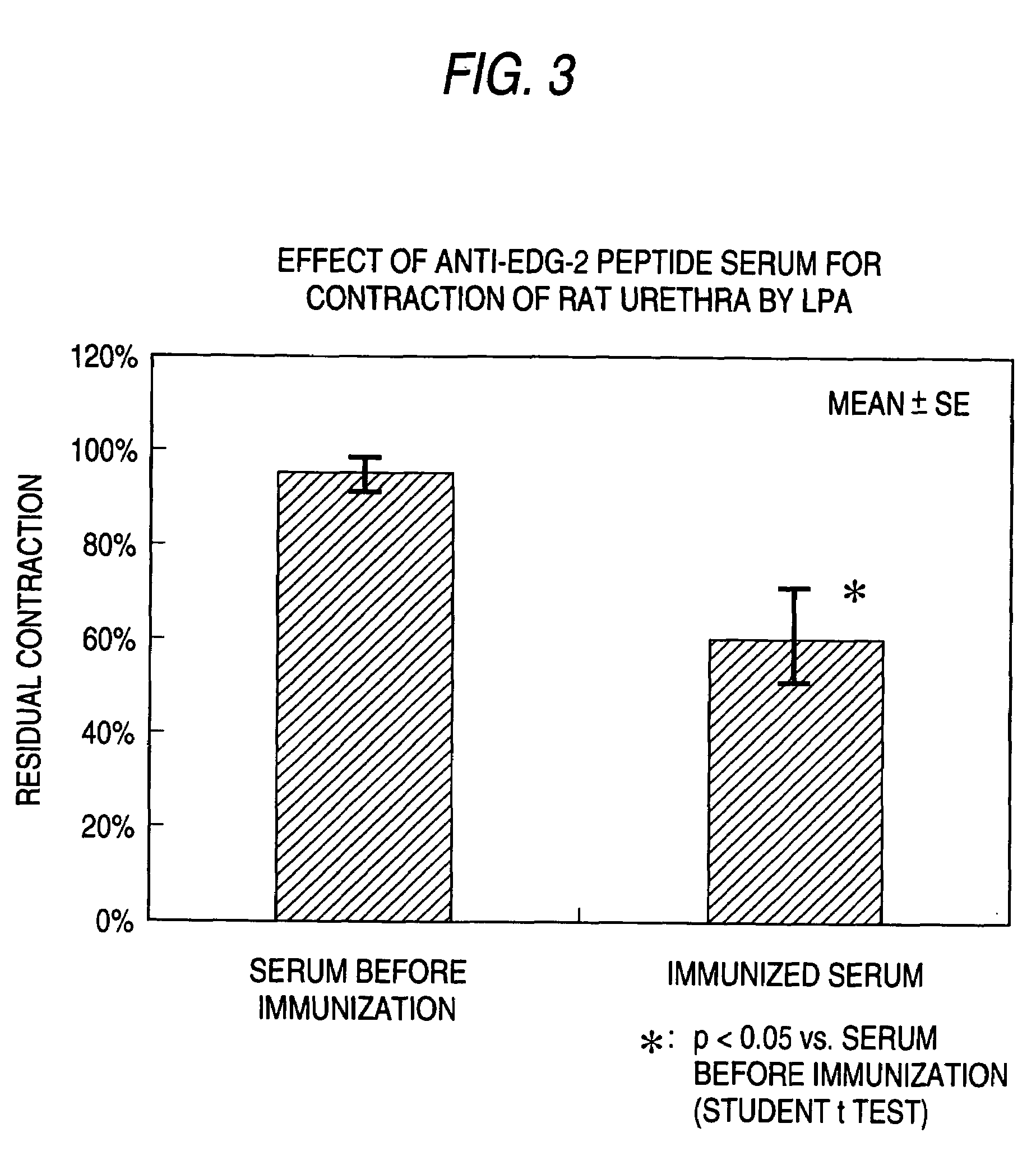
InactiveUS7300917B2Easy to prepareLow toxicityOrganic active ingredientsPeptide/protein ingredientsLPA ReceptorsDisease cause
A remedy and / or a preventive for a chronic disease which contains an EDG-2 antagonist. Because of binding to a subtype EDG-2 of LPA receptor, an EDG-2 antagonist is useful in treating and / or preventing chronic diseases (for example, diseases caused by the progress of chronic asthma, glomerular nephritis, obesity, arteriosclerosis, rheumatoid and atopic diseases) induced and made chronic by tissue cells whose proliferation is accelerated by LPA mediated by EDG-2.
Owner:ONO PHARMA CO LTD
Remedy for chronic disease
InactiveUS20060135577A1Effective preventionEffective treatmentBiocidePeptide/protein ingredientsLPA ReceptorsAntagonist
A remedy and / or a preventive for a chronic disease which contains an EDG-2 antagonist. Because of binding to a subtype EDG-2 of LPA receptor, an EDG-2 antagonist is useful in treating and / or preventing chronic diseases (for example, diseases caused by the progress of chronic asthma, glomerular nephritis, obesity, arteriosclerosis, rheumatoid and atopic diseases) induced and made chronic by tissue cells whose proliferation is accelerated by LPA mediated by EDG-2.
Owner:ONO PHARMA CO LTD
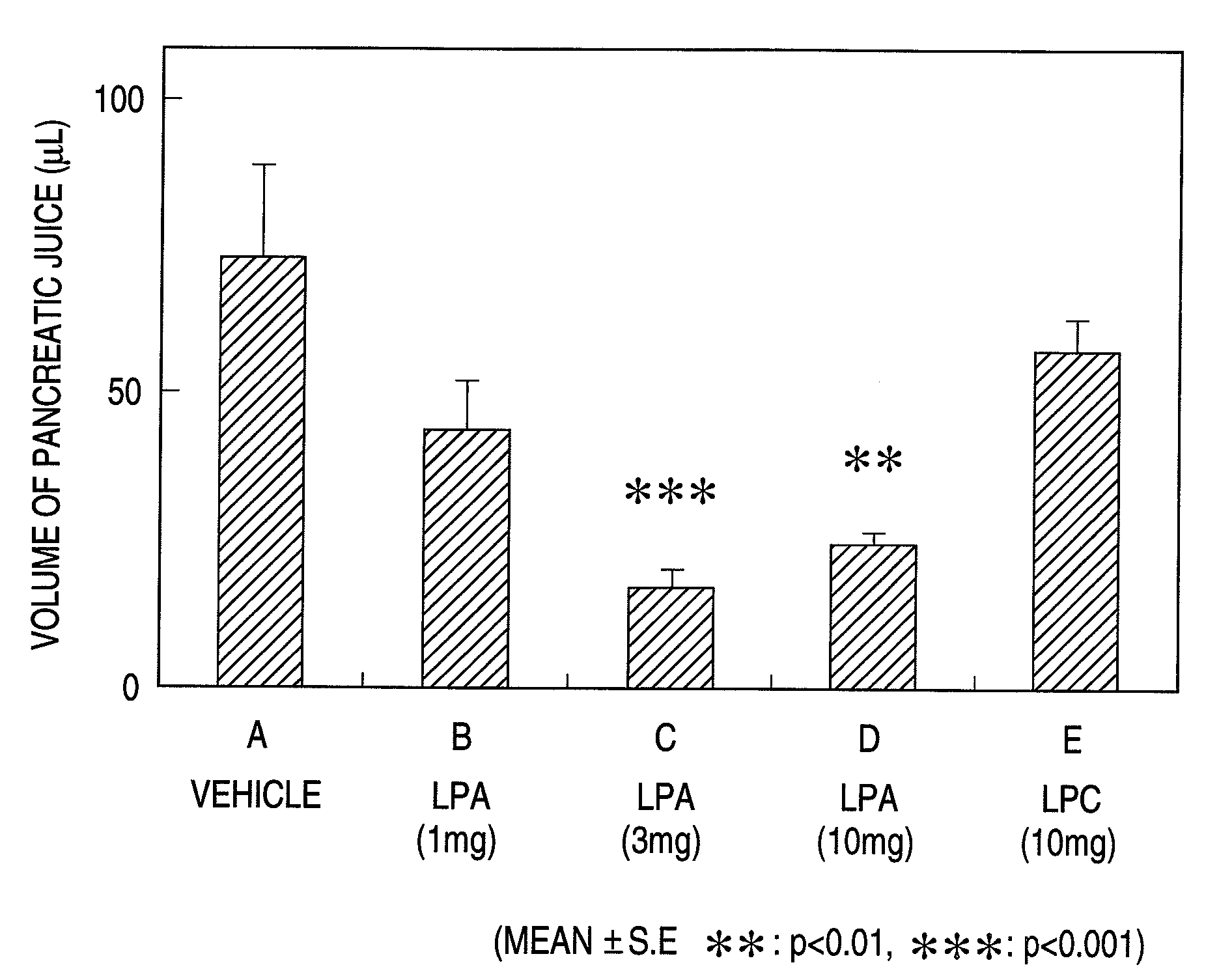
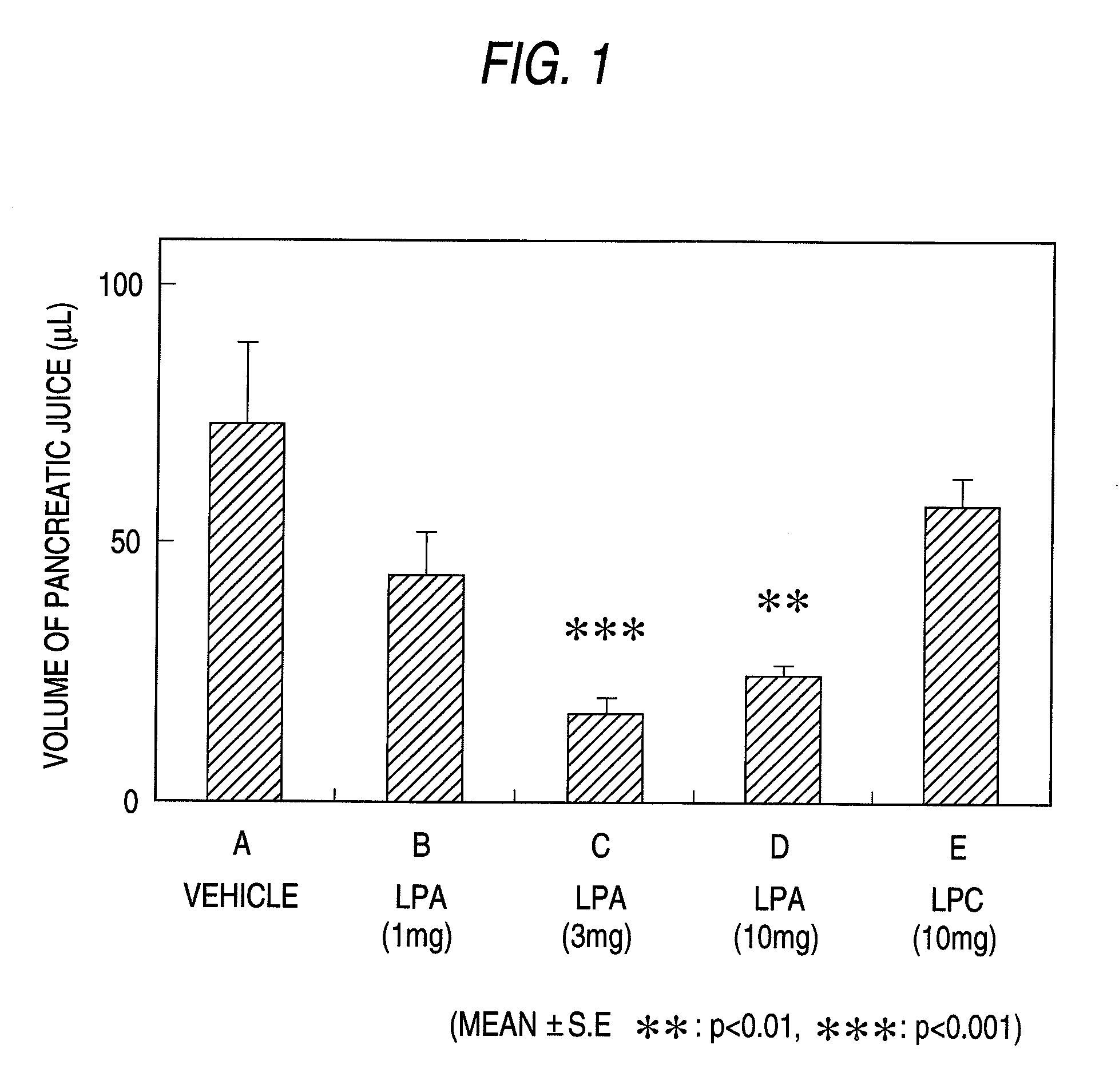
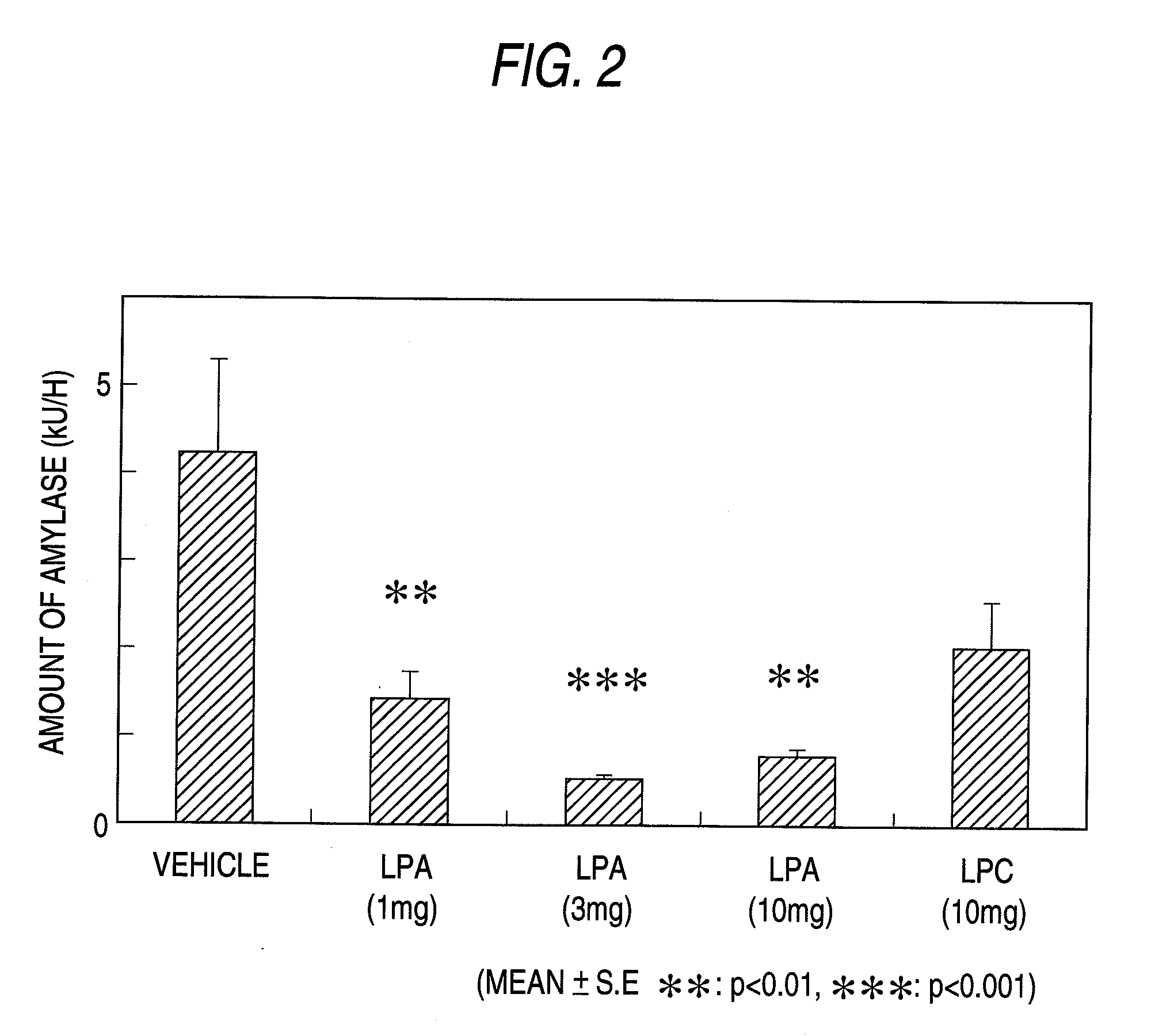
Pharmaceutical Composition for Regulation of Pancreatic Juice Secretion Comprising a LPA Receptor Modulator
Pharmaceutical composition for regulation of pancreatic juice secretion characterized by comprising a lisophosphatidic acid (LPA) receptor modulator. Since an LPA receptor modulator has an effect of regulating the secretion of pancreatic juice, a compound acting on this receptor is useful in treating diseases in association with disorders in pancreatic juice secretion. For example, an LPA receptor agonist is useful in treating and / or preventing pancreatic diseases and obesity, while an LPA receptor antagonist is useful in treating and / or preventing maldigestion, constipation, diarrhea and cibophobia.
Owner:ONO PHARMA CO LTD
Carbamoyloxymethyl triazole cyclohexyl acids as LPA antagonists
The present invention provides compounds of Formula (I):or stereoisomers, tautomers, pharmaceutically acceptable salts, solvates or prodrugs thereof, wherein all the variables are as defined herein. These compounds are selective LPA receptor inhibitors.
Owner:BRISTOL MYERS SQUIBB CO
Azole compound
Azole compounds represented by formula I:wherein ring A is isoxazole and the like, R1 is a substituted or unsubstituted aryl group and the like, R2 is a hydrogen atom and the like, and R3 is a substituted or unsubstituted alkyl group and the like, and pharmaceutically acceptable salts thereof inhibit the physiological activity of lysophosphatidic acid (LPA), and are useful as for the prophylaxis or treatment of diseases in which inhibition of the physiological activity of LPA is useful for the prophylaxis or treatment thereof, such as diseases involving the LPA receptor.
Owner:AJINOMOTO CO INC
Triazole n-linked carbamoyl cyclohexyl acids as lpa antagonists
Owner:BRISTOL MYERS SQUIBB CO
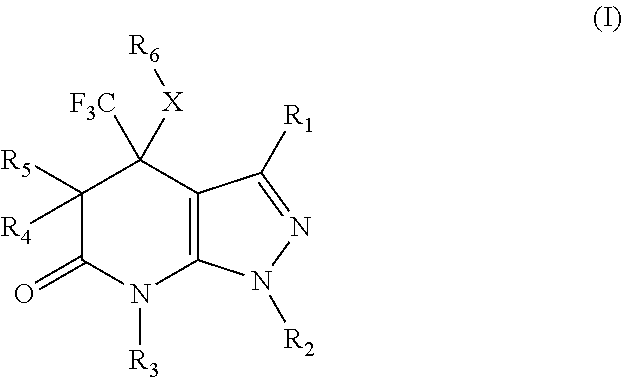
Pyrazolopyridinone derivatives as lpa receptor antagonists
The present invention relates to novel pyrazolopyridinone derivatives according to formula (I) and a process of manufacturing thereof. These pyrazolopyridinone derivatives can be used as LPA receptor antagonists for the treatment of various herein disclosed diseases.
Owner:MERCK PATENT GMBH
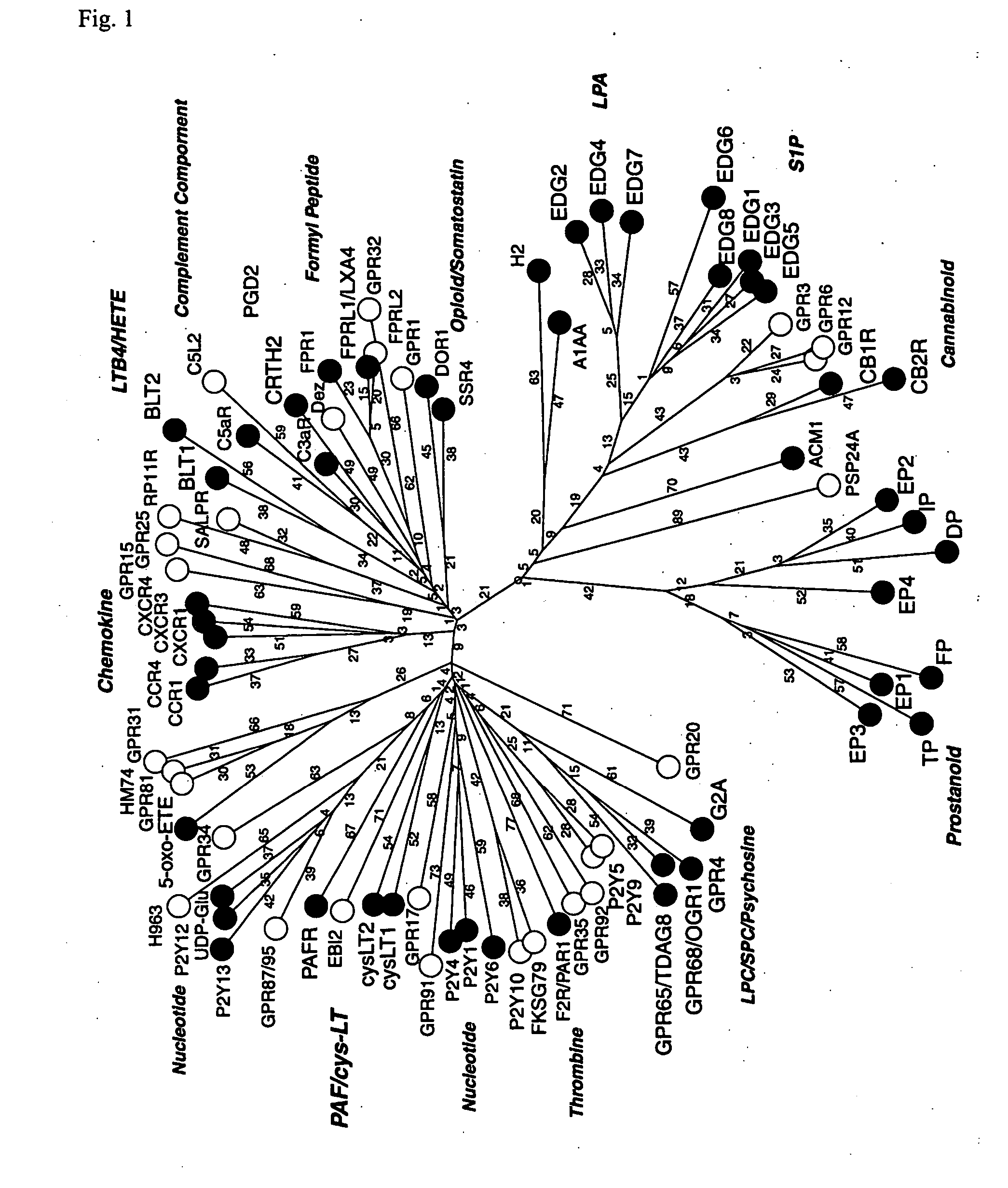
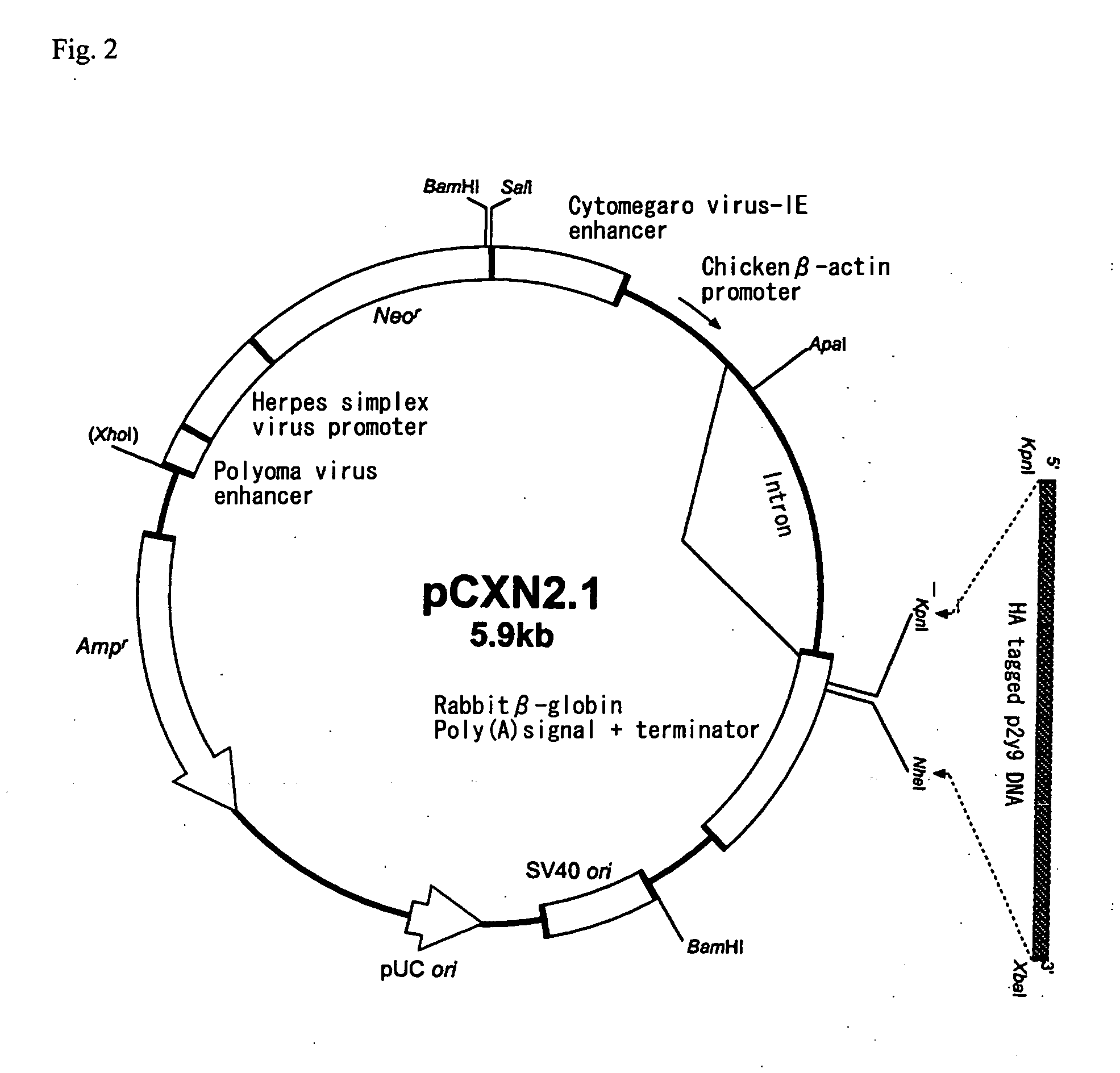
Novel lysophosphatidic acid receptor
InactiveUS20060264361A1Peptide/protein ingredientsMicrobiological testing/measurementLPA ReceptorsAgonist
It is intended to provide a novel receptor of LPA and a method of screening a drug such as an LPA receptor antagonist using the same. Use as a lysophosphatidic acid (LPA) receptor comprising a G protein-coupled protein p2y9. More specifically, use of the G protein-coupled protein p2y9 as a lysophosphatidic acid (LPA) receptor. A method of screening an agonist or an antagonist to the LPA receptor as described above by using the receptor.
Owner:JAPAN SCI & TECH CORP
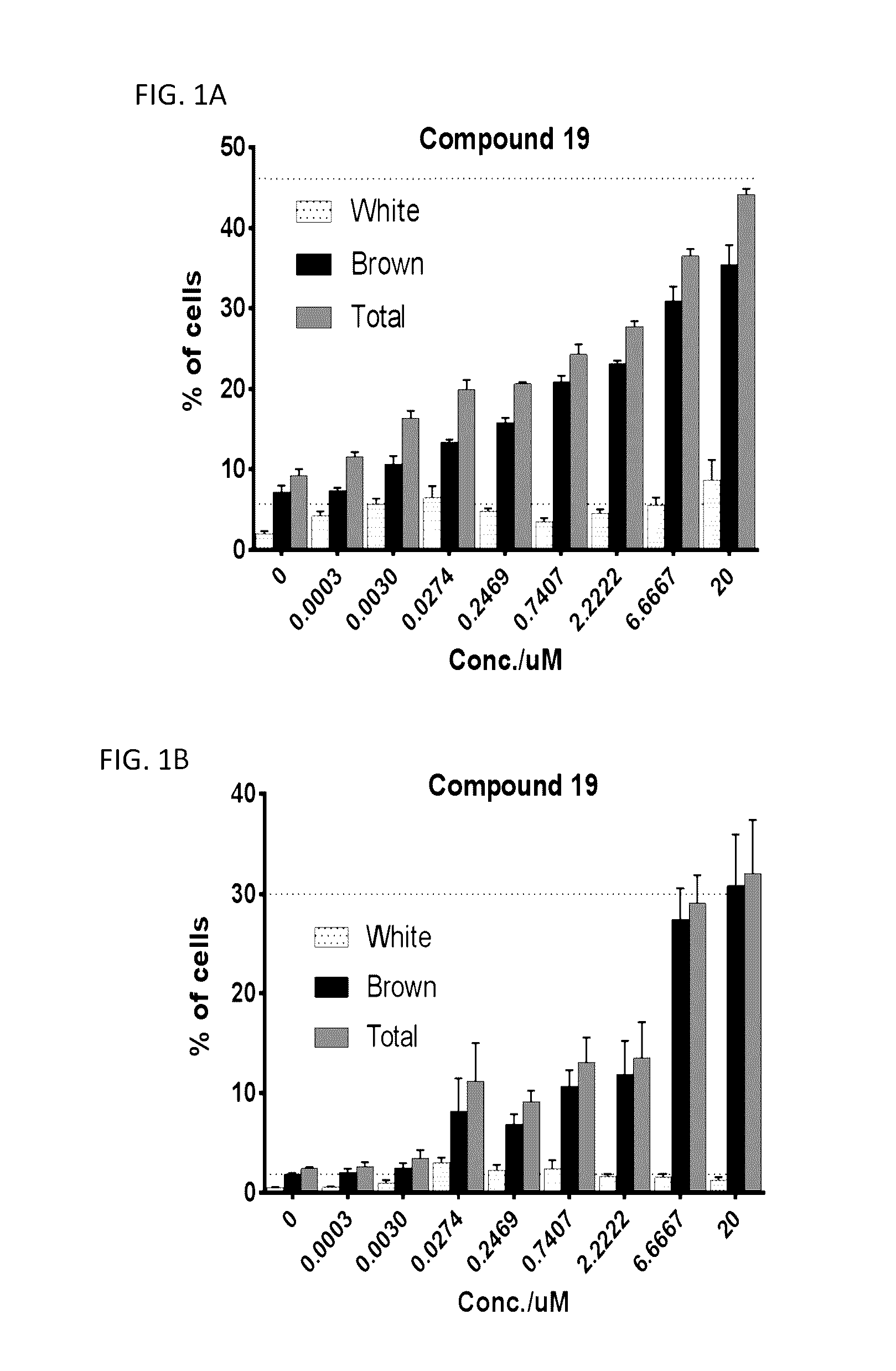
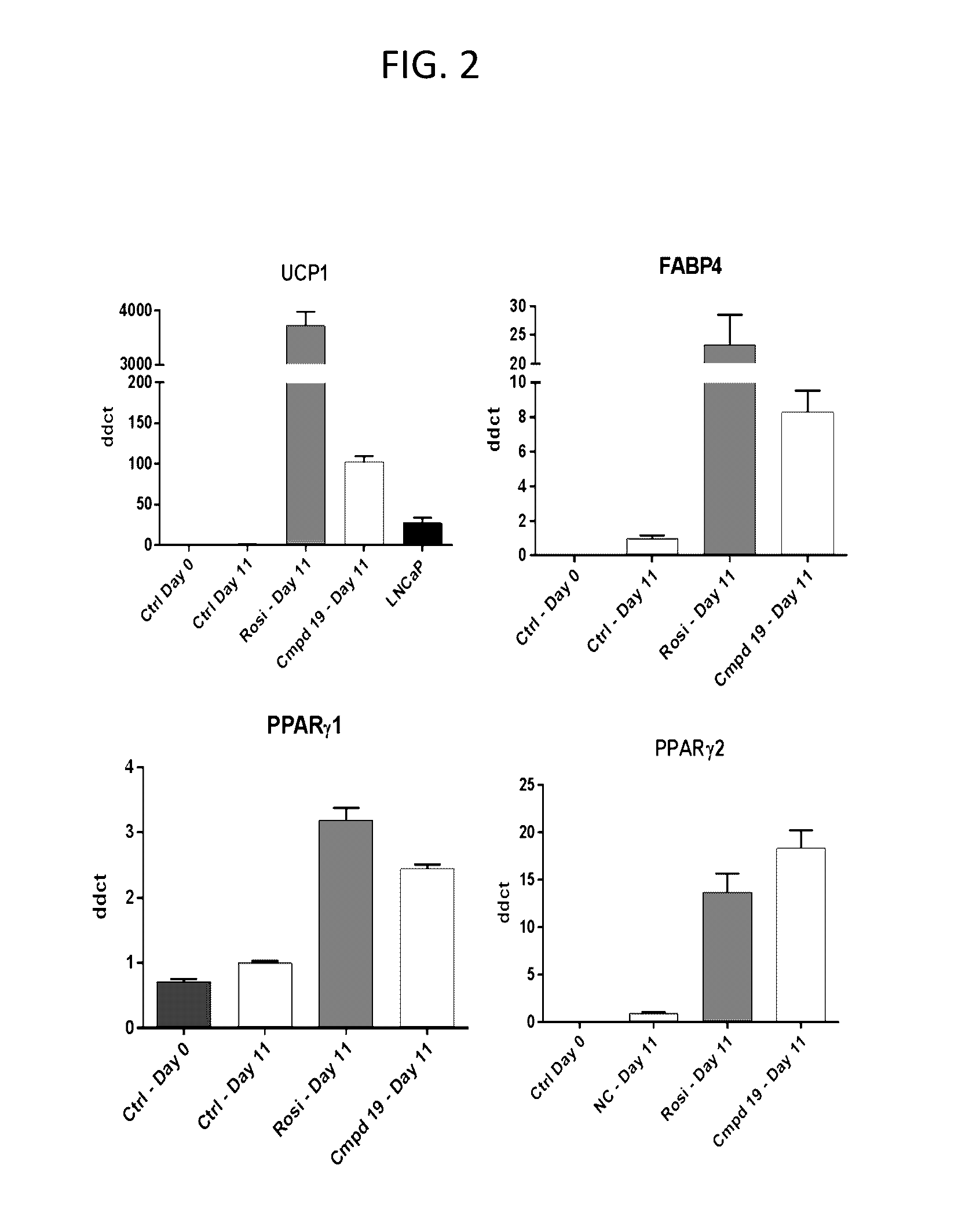
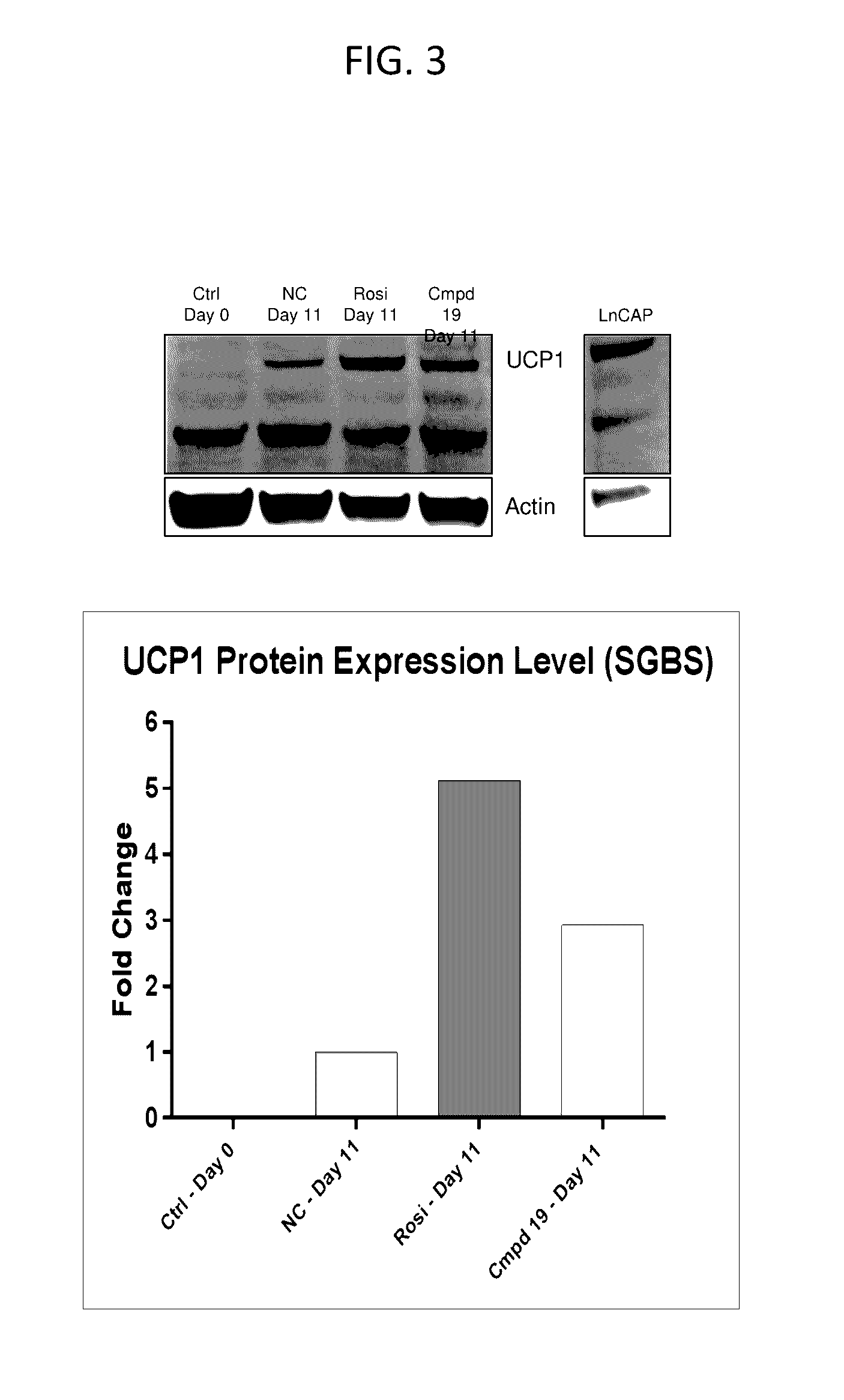
Lpa receptor modulators for brown fat differentiation
InactiveUS20170042915A1Increase differentiationIncreasing brown fat differentiationHeterocyclic compound active ingredientsLPA ReceptorsMetabolite
Described herein is a method of increasing brown fat differentiation or increasing energy expenditure in a subject in need thereof, the method comprising administering to the subject an LPA receptor modulator, or a pharmaceutically acceptable salt, solvate, polymorph, prodrug, metabolite, deuteride, N-oxide, stereoisomer, or isomer thereof.
Owner:THE SCRIPPS RES INST
Vinyl phosphonate lysophosphatidic acid receptor antagonists
ActiveUS20090197835A1Improve usabilityReduce harmOrganic active ingredientsBiocideLPA ReceptorsAntagonist
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Use for glycolipoprotein gintonin, isolated and identified from ginseng, as a natural medical-plant derived ligand
InactiveUS20140234868A1Trouble growingPromoting various calcium-dependent biologicalNervous disorderAntipyreticLPA ReceptorsNervous system
The present invention relates to glycolipoprotein gintonin, isolated and identified from ginseng, as a natural medicinal-plant-derived ligand acting on LPA1 (lysophosphatidic acid; 1- or 2-acyl-sn-glycerol-3-phosphate), LPA2, LPA3, LPA4 and LPA5 receptors whose efficacy is exhibited physiologically / pharmaceutically via an interaction with subset receptors [LPA1(edg-2), LPA2(edg-4), LPA3(edg-7), LPA4, PLA5] in the EDG (endothelial differentiation gene) family in G protein-coupled receptors (GPCRs) present in the cell membranes of animals including humans. The gintonin of the present invention can be used to advantage in the prevention and treatment of various diseases arising from reduced calcium concentration and various physiological activities and pharmaceutical activities dependent on calcium, since the gintonin of the present invention interacts with LPA receptors so as to activate a series of signal transmission processes and temporarily induce an increase in free Ca2+ in the cytoplasm, and a temporary increase in the intracellular calcium concentration gives rise to a temporary increase in the intracellular calcium concentration in various organs including, inter alia, those of the nervous system, cardiovascular system, endocrine system, reproductive system, digestive system and immune system when the LPA receptors are expressed, with physiological activity being exhibited.
Owner:KONKUK UNIV IND COOP CORP
Isoxazole o-linked carbamoyl cyclohexyl acids as LPA antagonists
Owner:BRISTOL MYERS SQUIBB CO
Cyclohexyl acid triazole azines as LPA antagonists
Owner:BRISTOL MYERS SQUIBB CO
Cyclohexyl acid triazole azines as lpa antagonists
ActiveUS20210230143A1Organic active ingredientsOrganic chemistryLPA ReceptorsPharmaceutical medicine
Owner:BRISTOL MYERS SQUIBB CO
Cyclohexyl acid pyrazole azoles as lpa antagonists
ActiveUS20210078982A1Organic active ingredientsOrganic chemistryLPA ReceptorsPharmaceutical medicine
Owner:BRISTOL MYERS SQUIBB CO
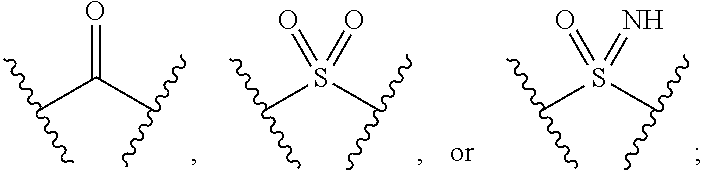
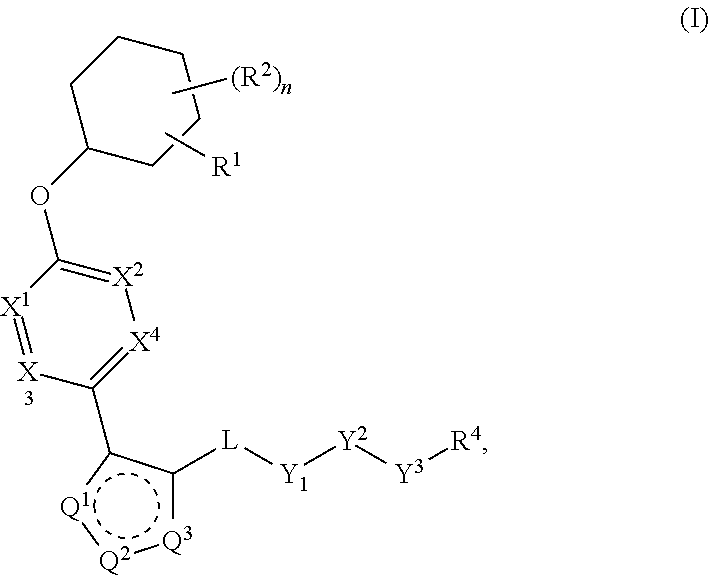
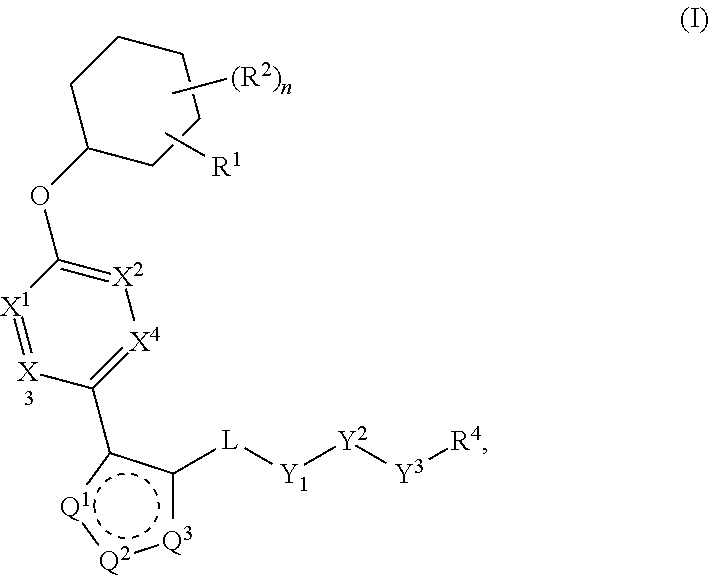
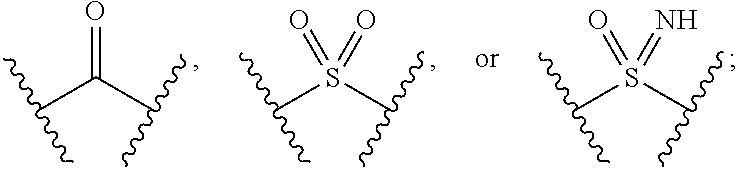
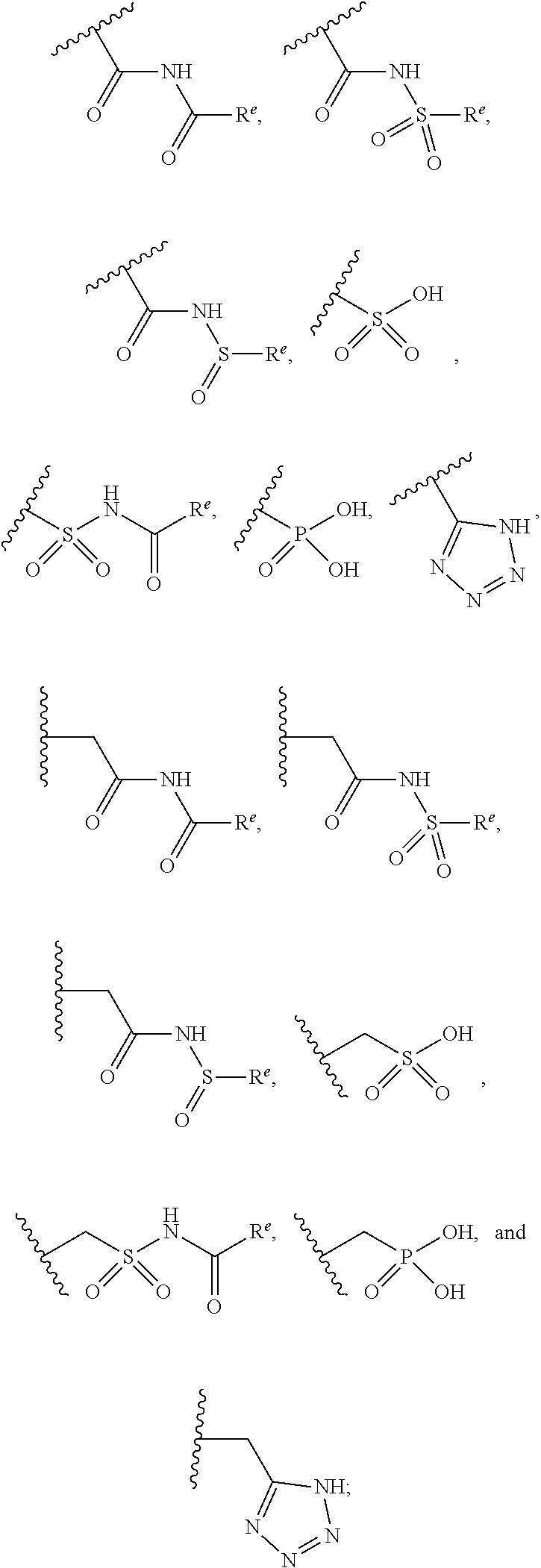
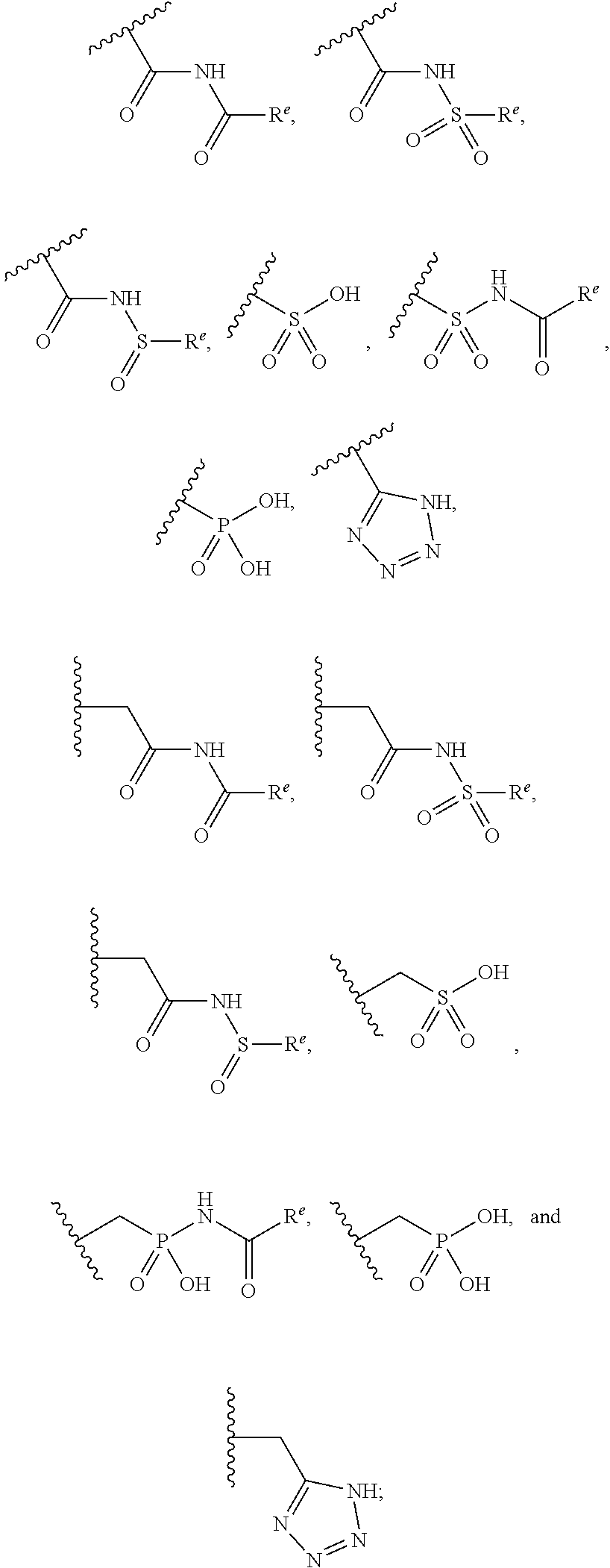
Pyrazole N-Linked Carbamoyl Cyclohexyl Acids as LPA Antagonists
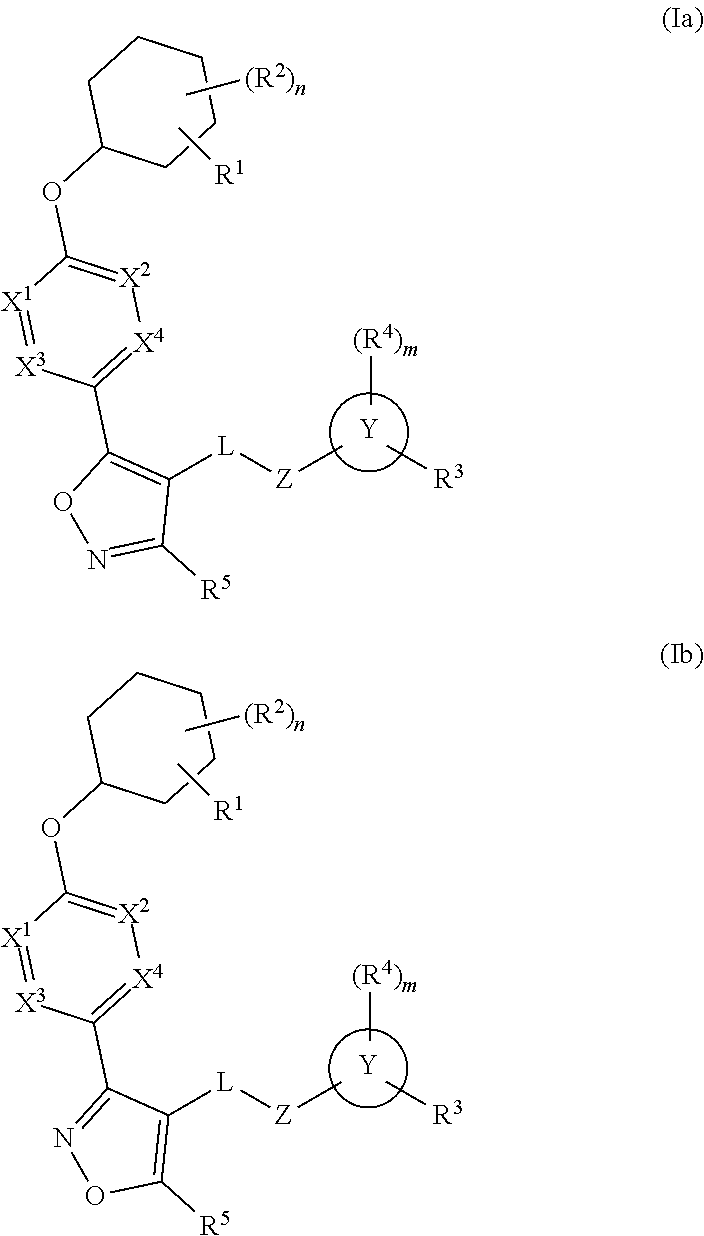
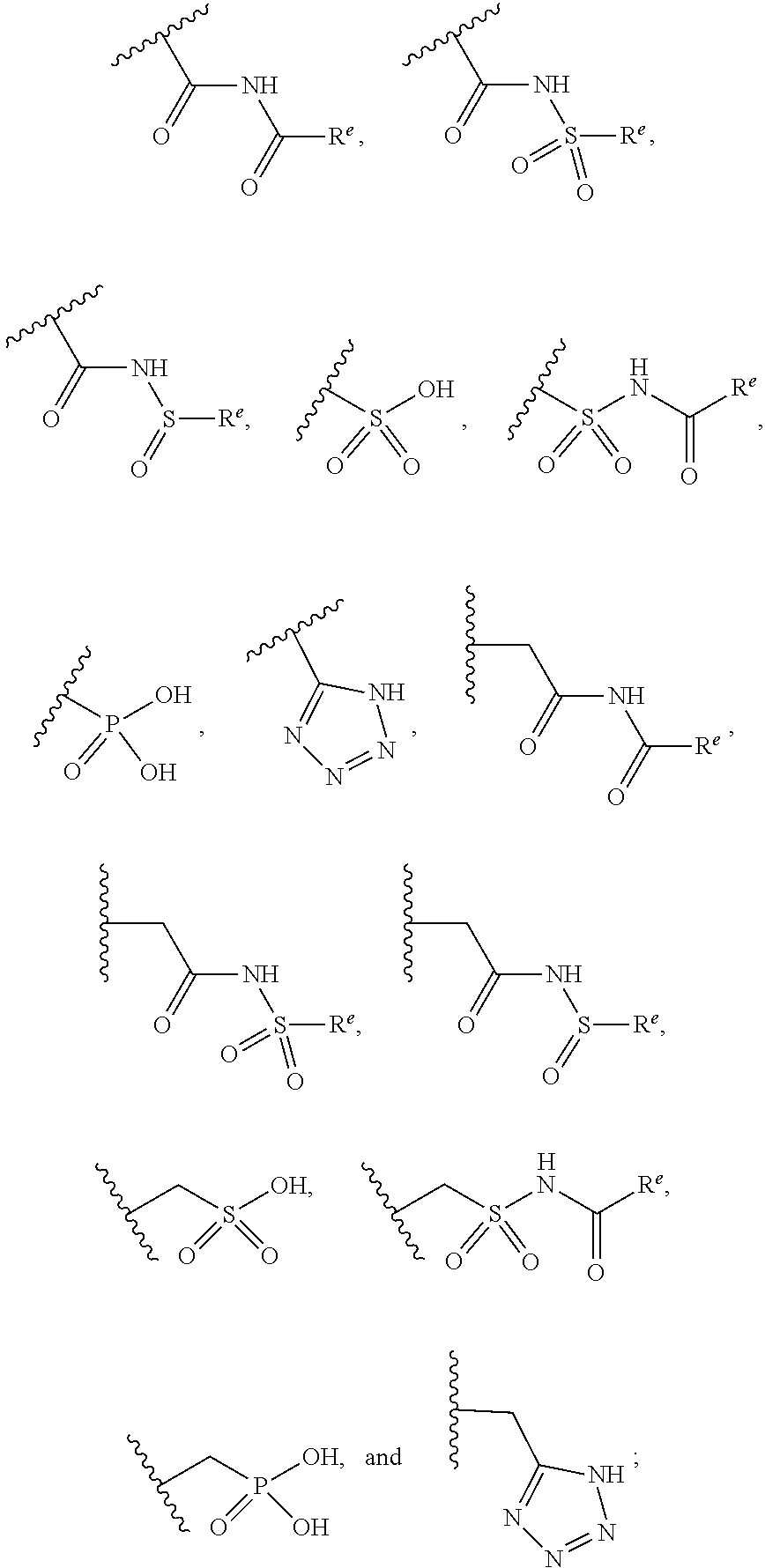
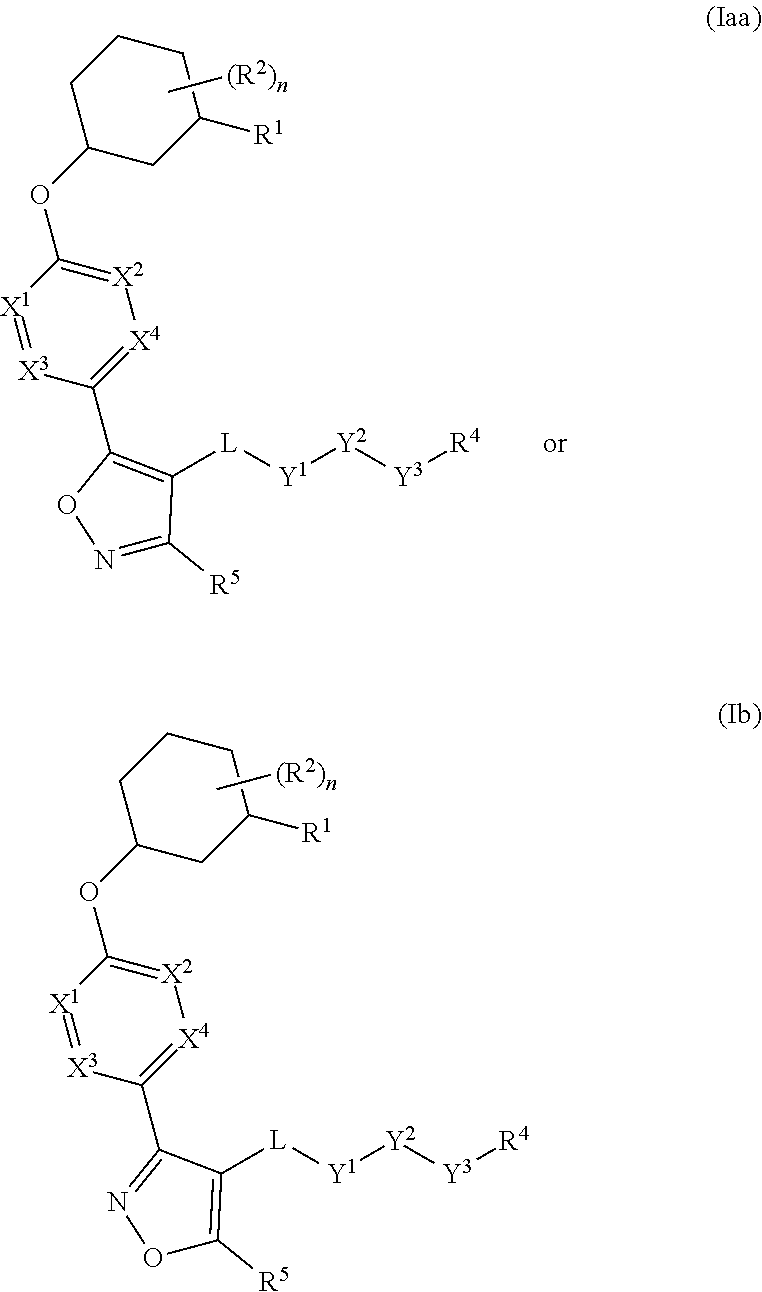
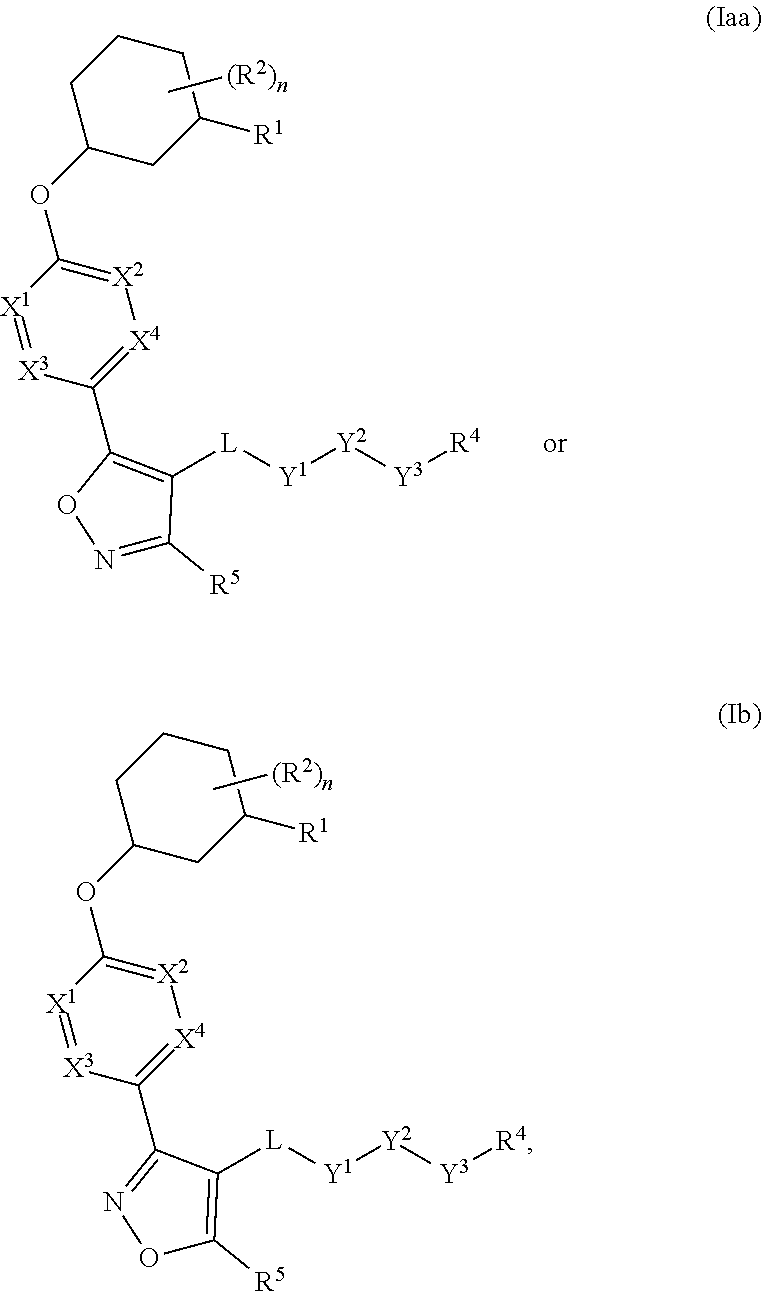
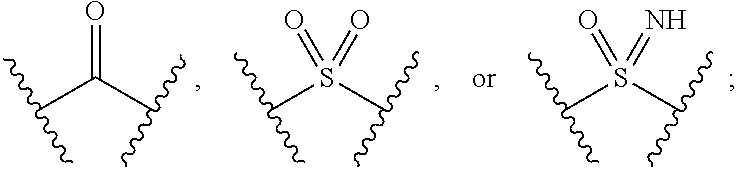
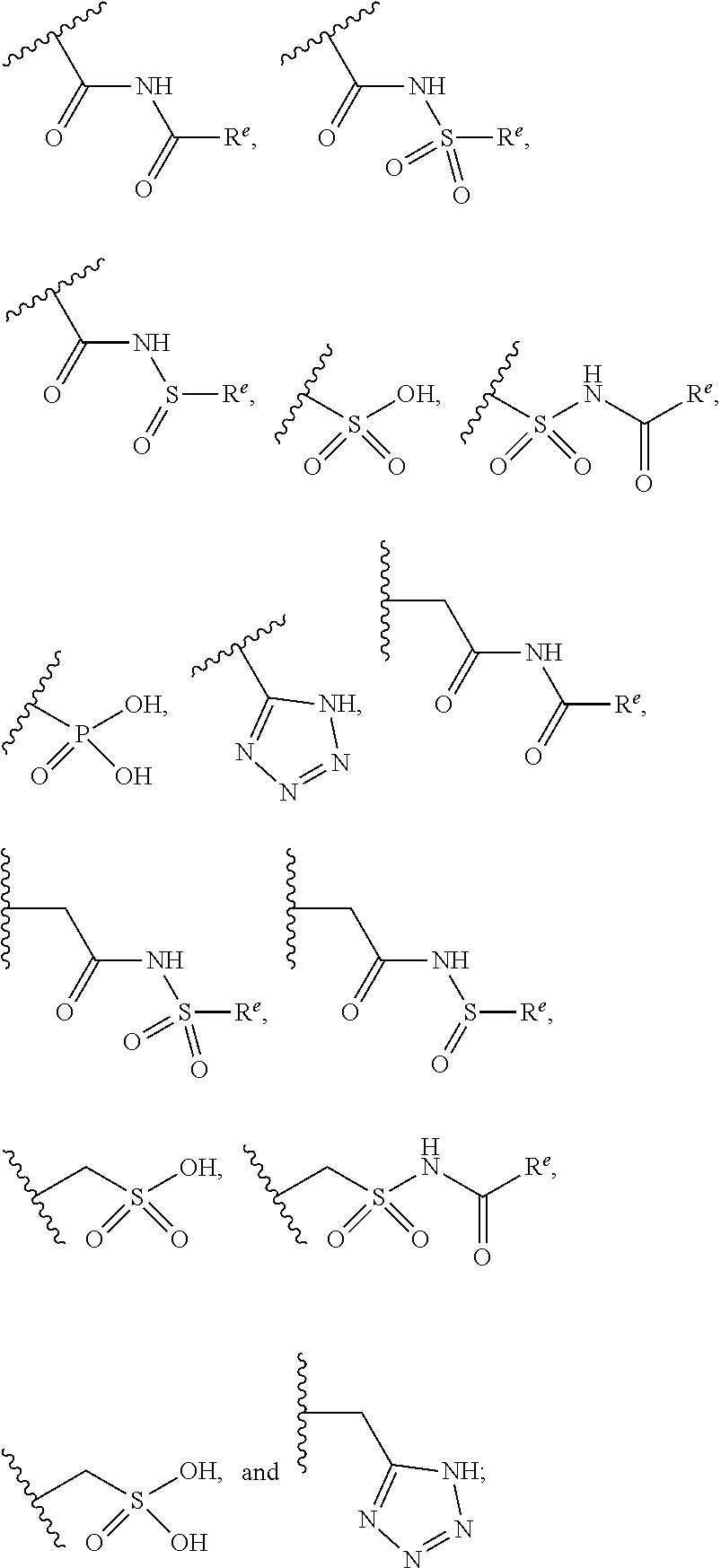
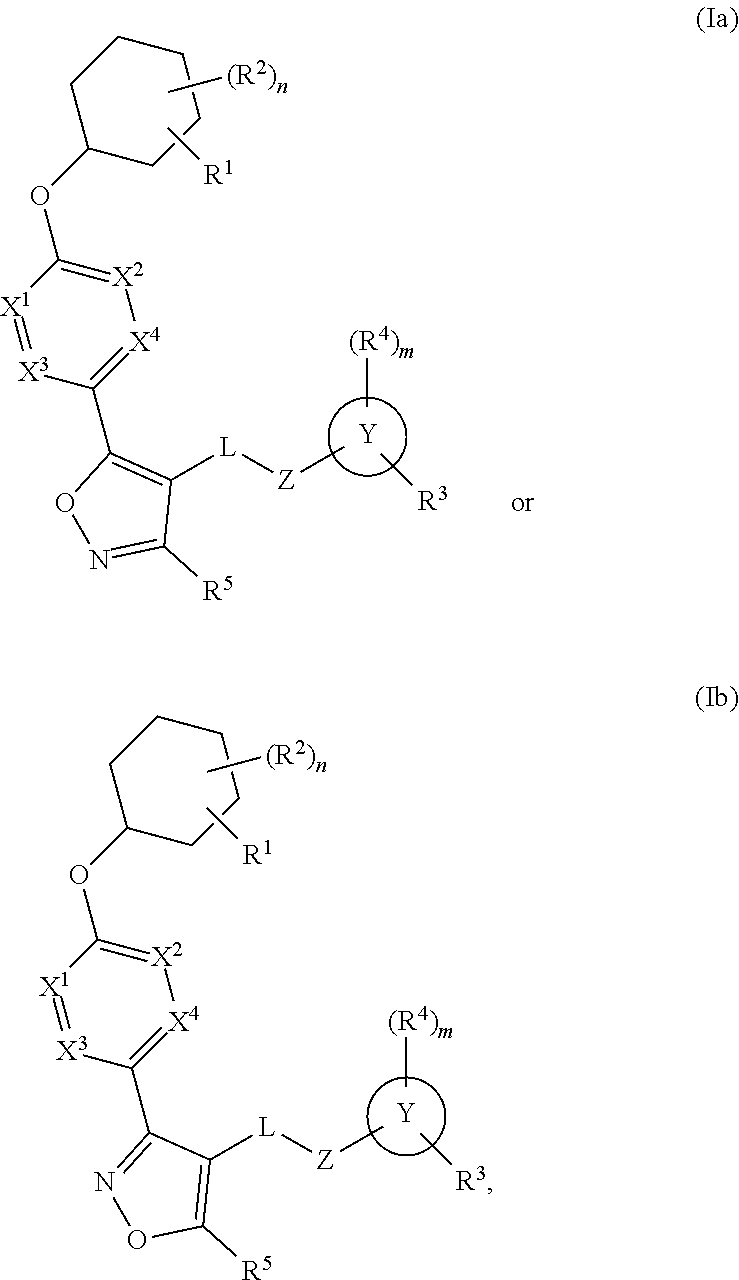
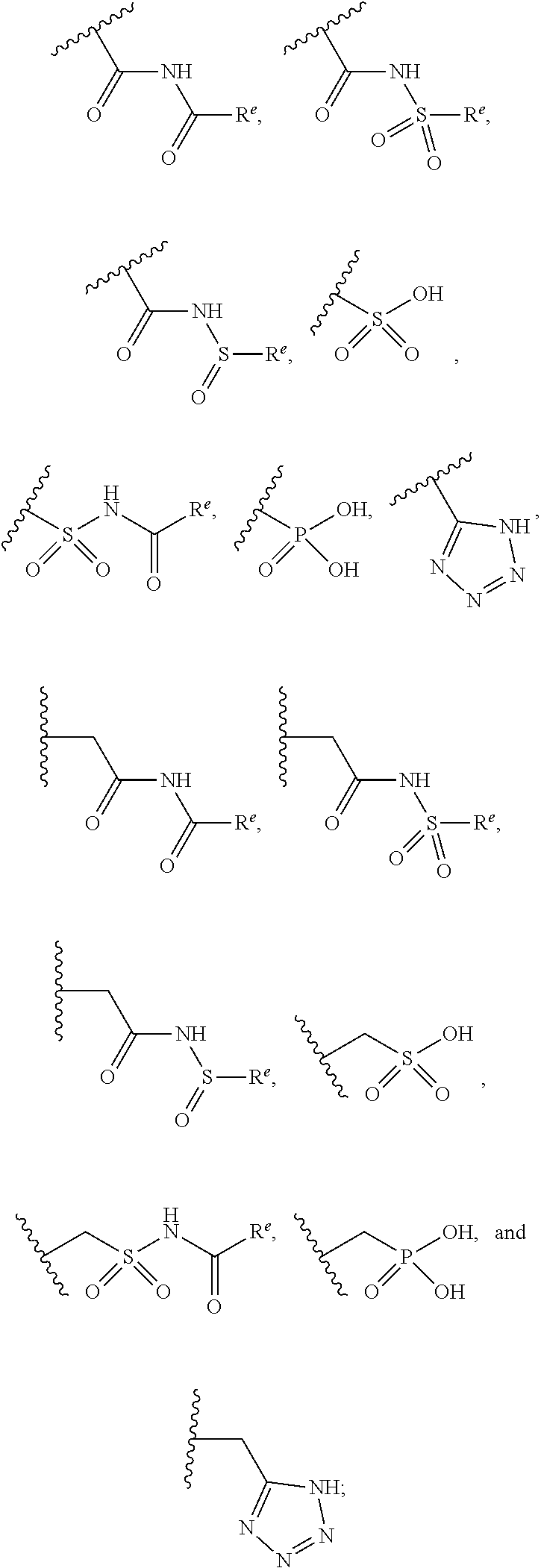
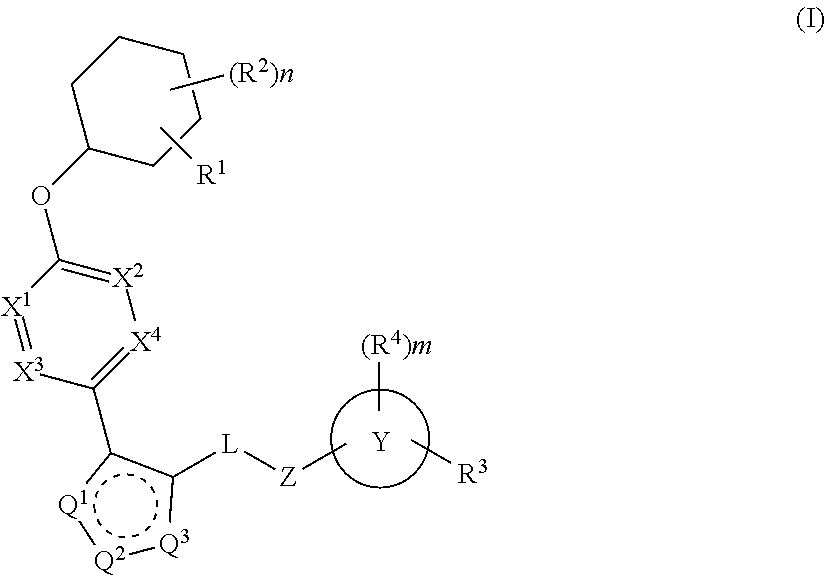
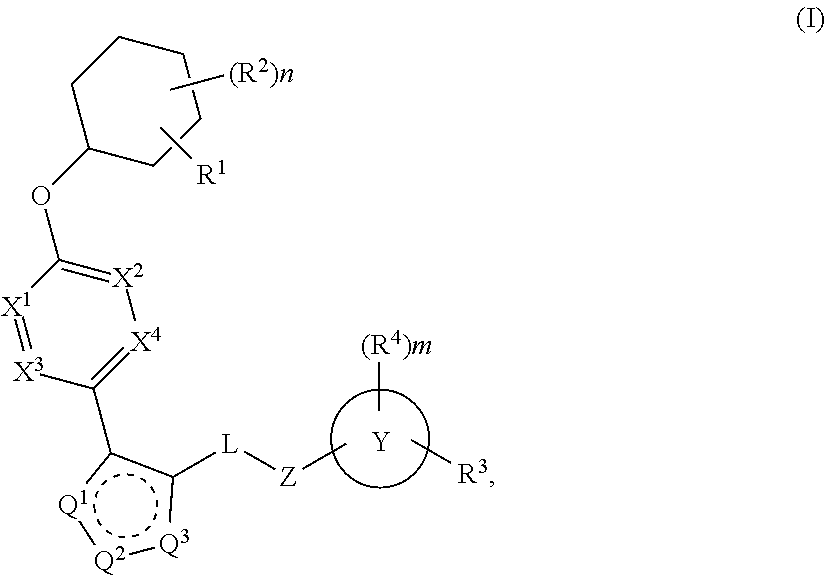
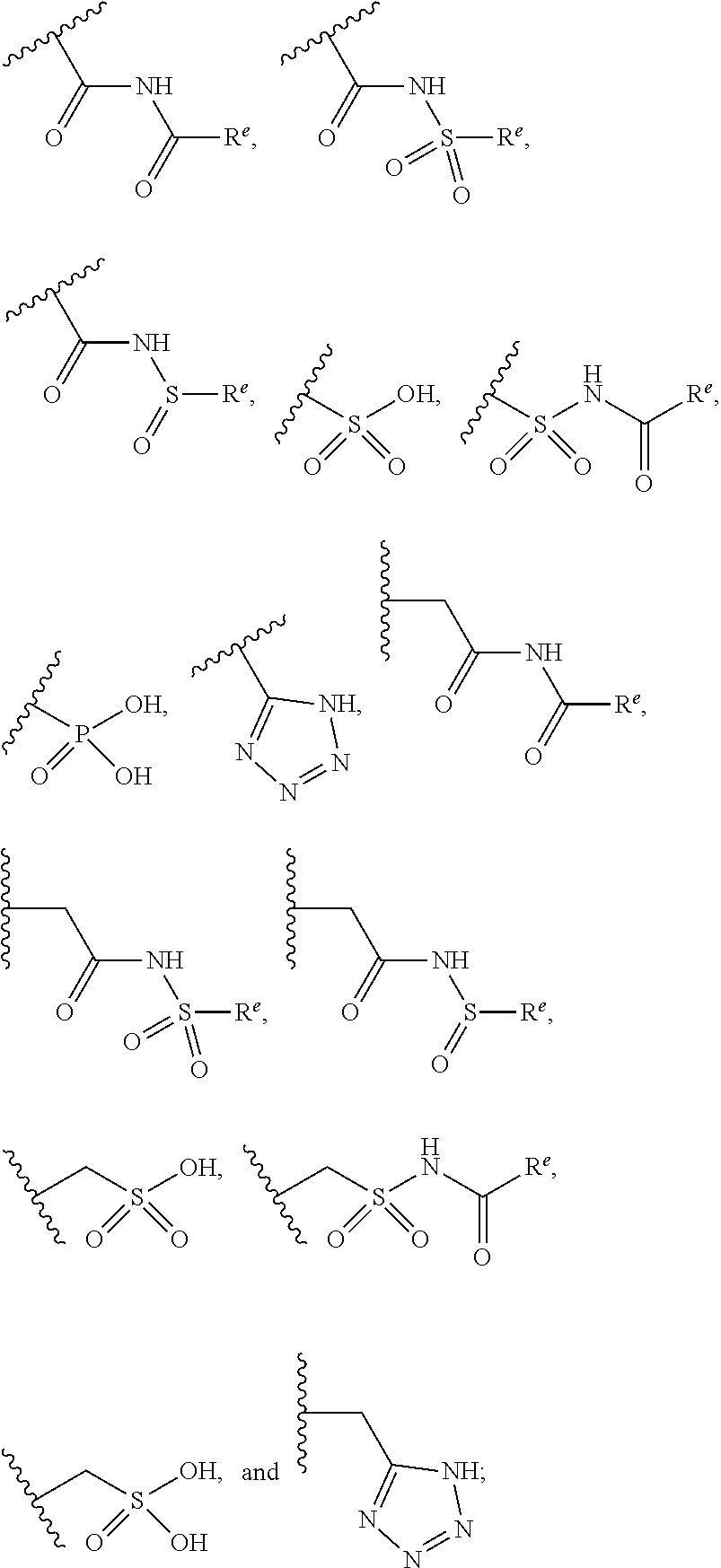
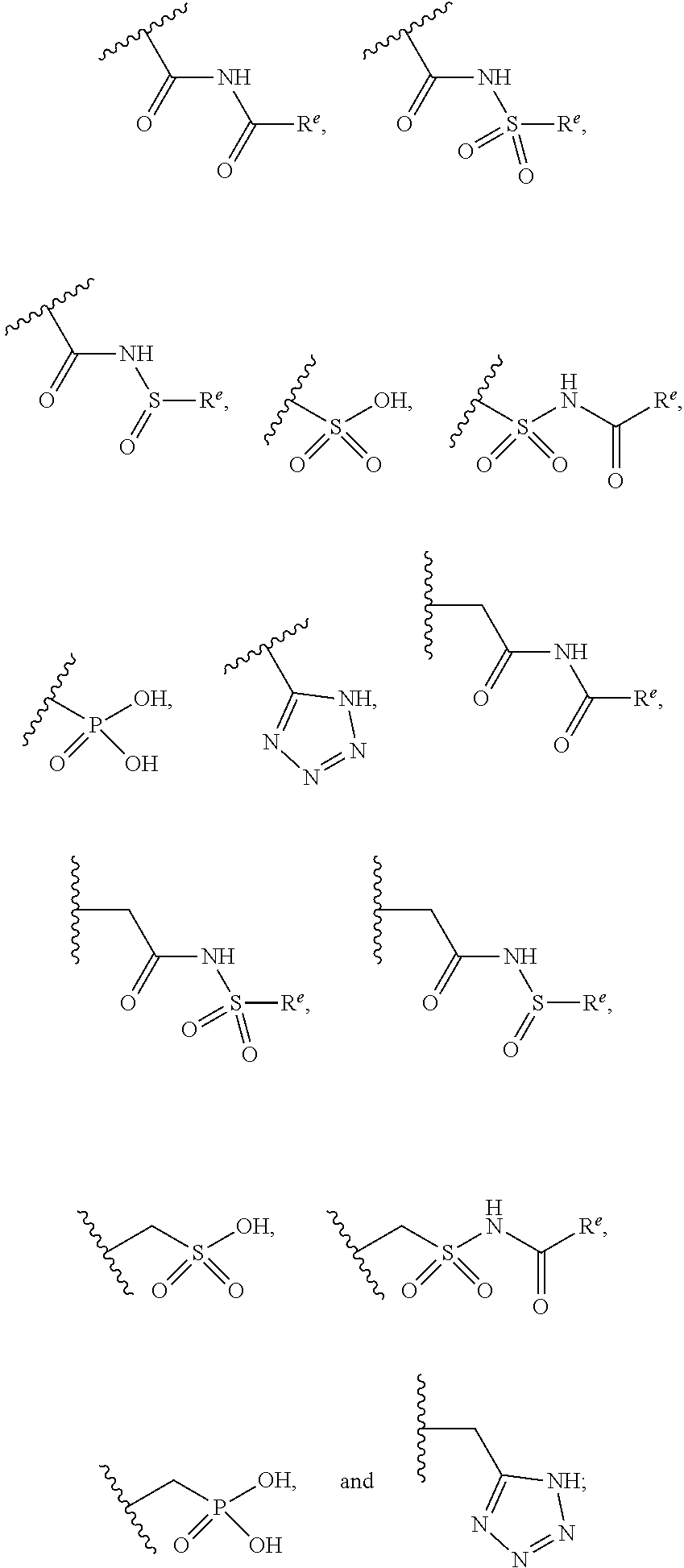
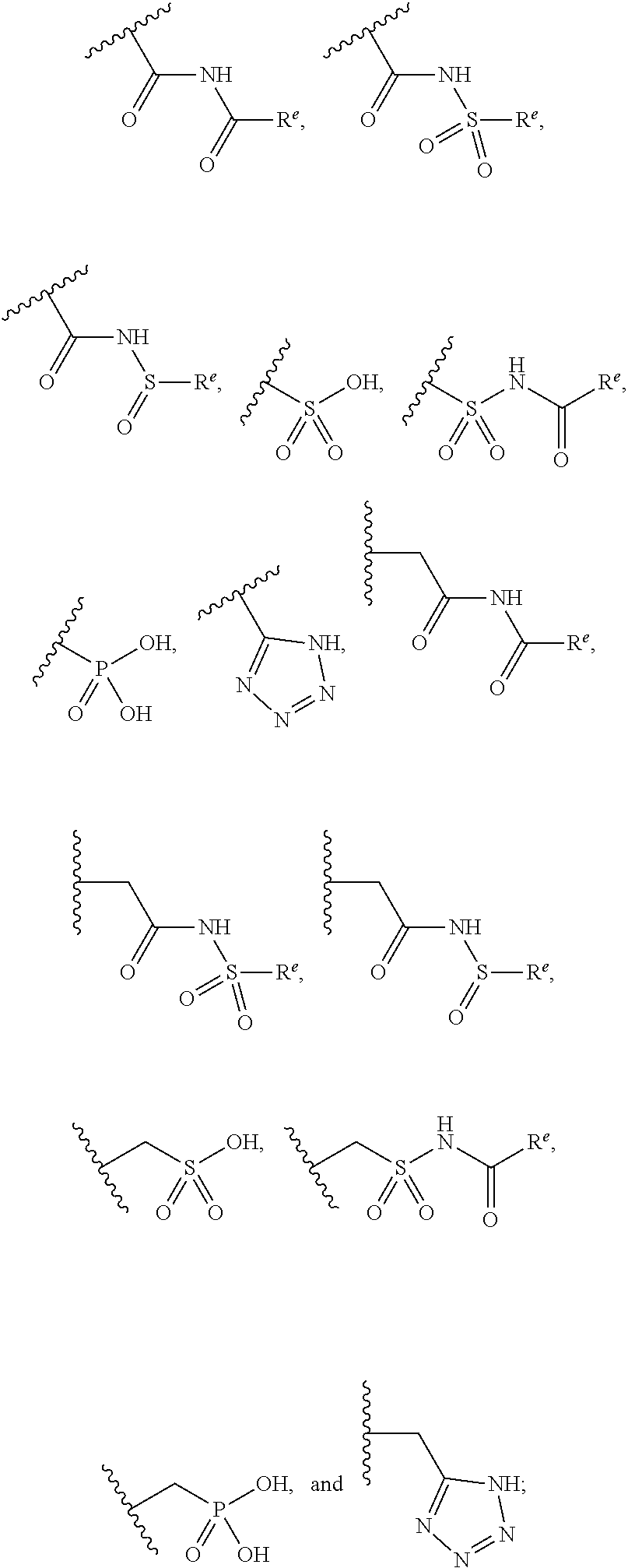
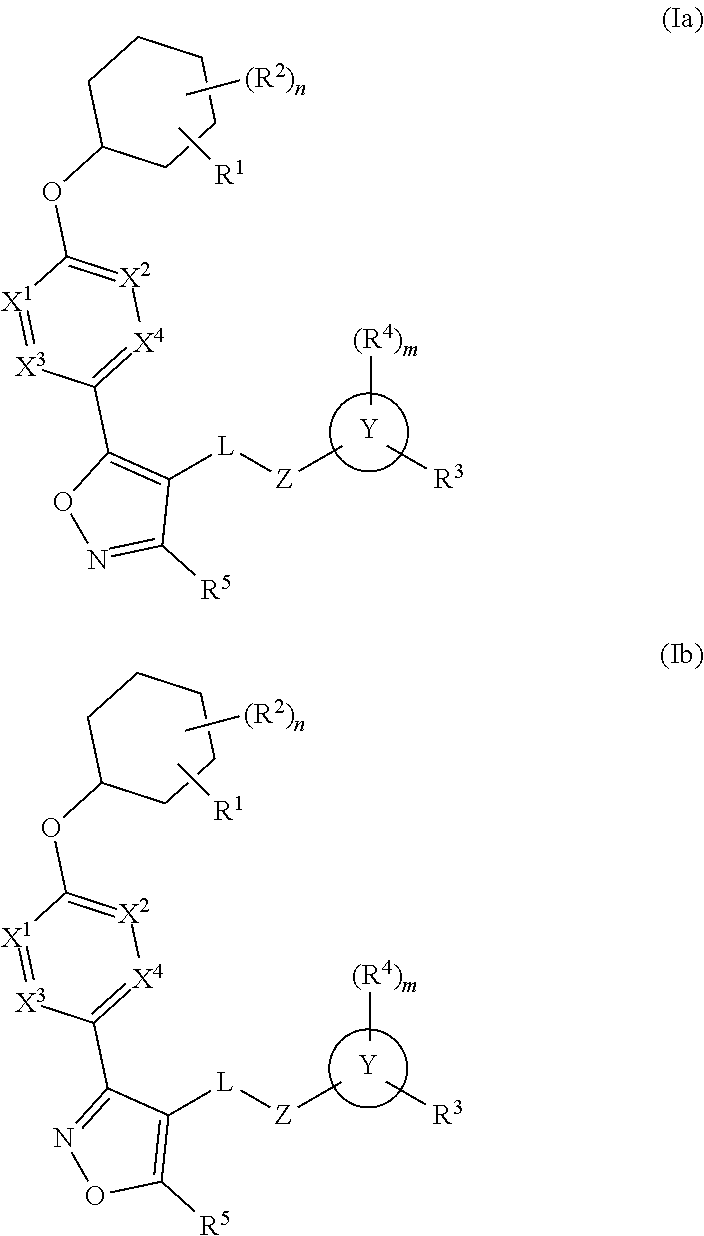
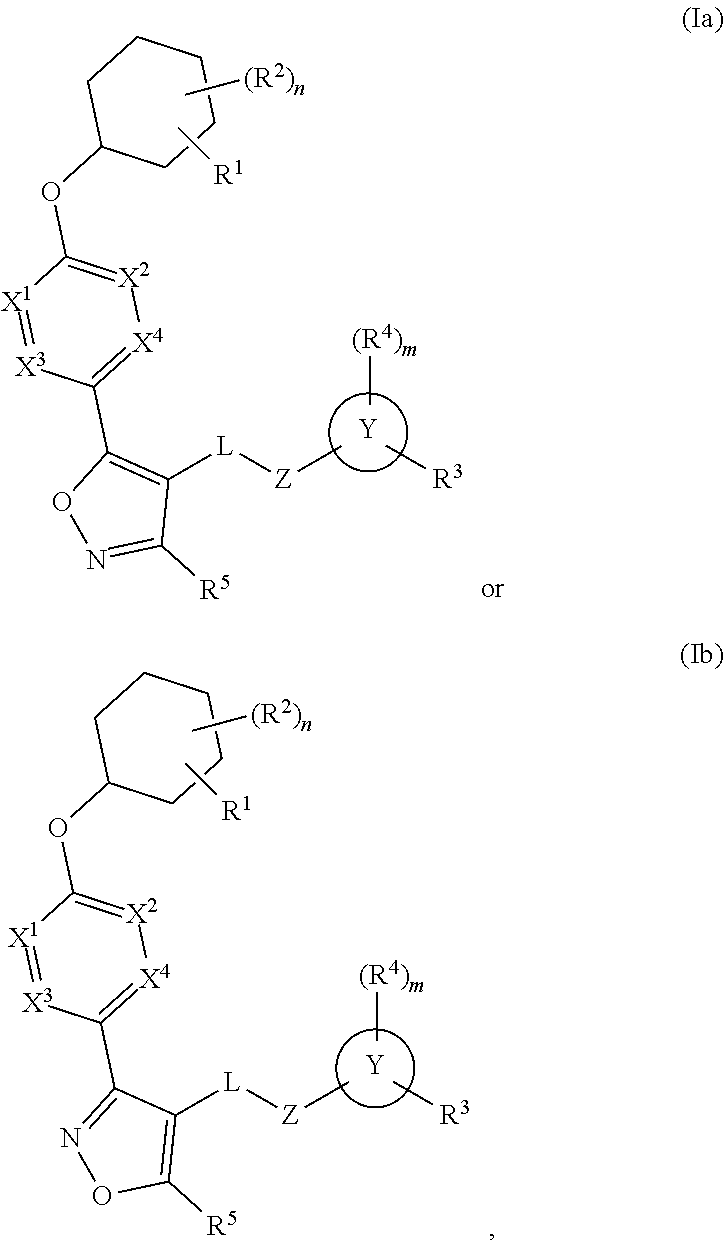
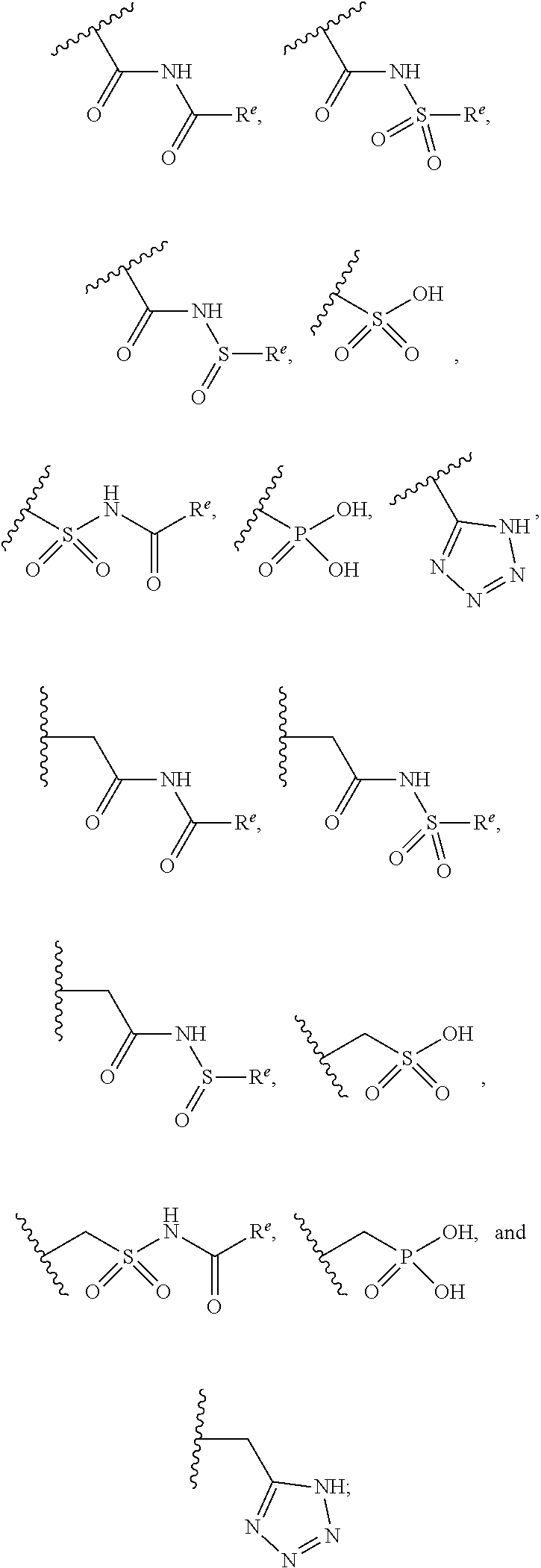
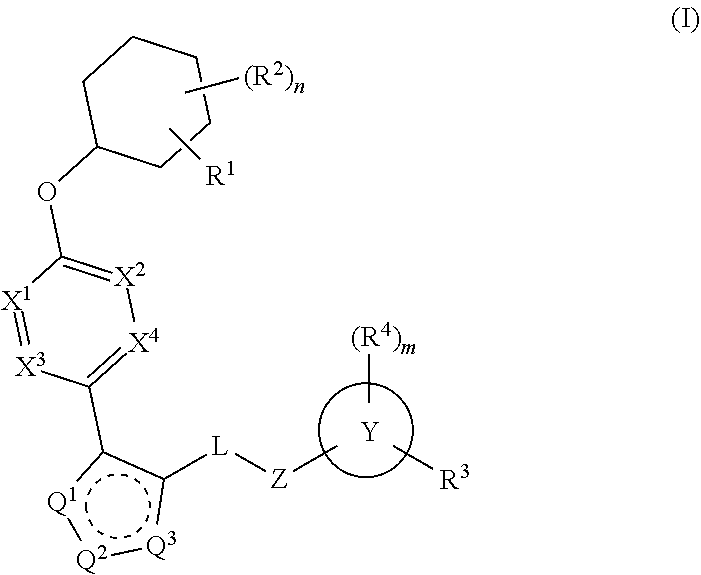
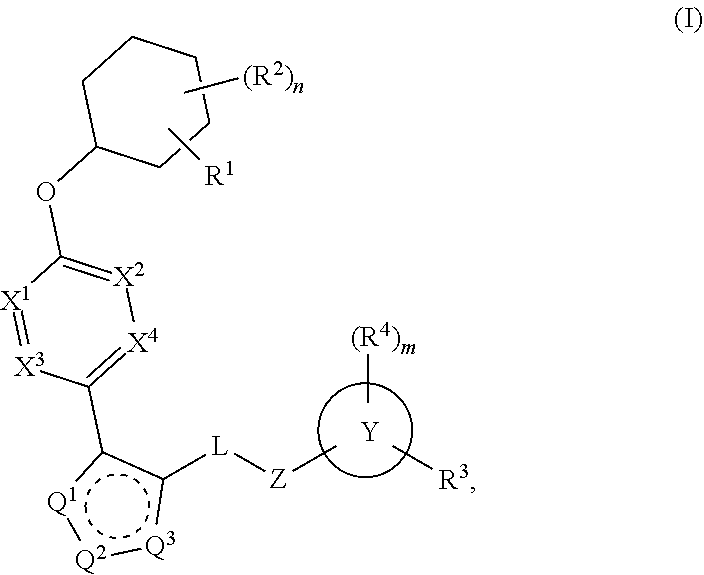
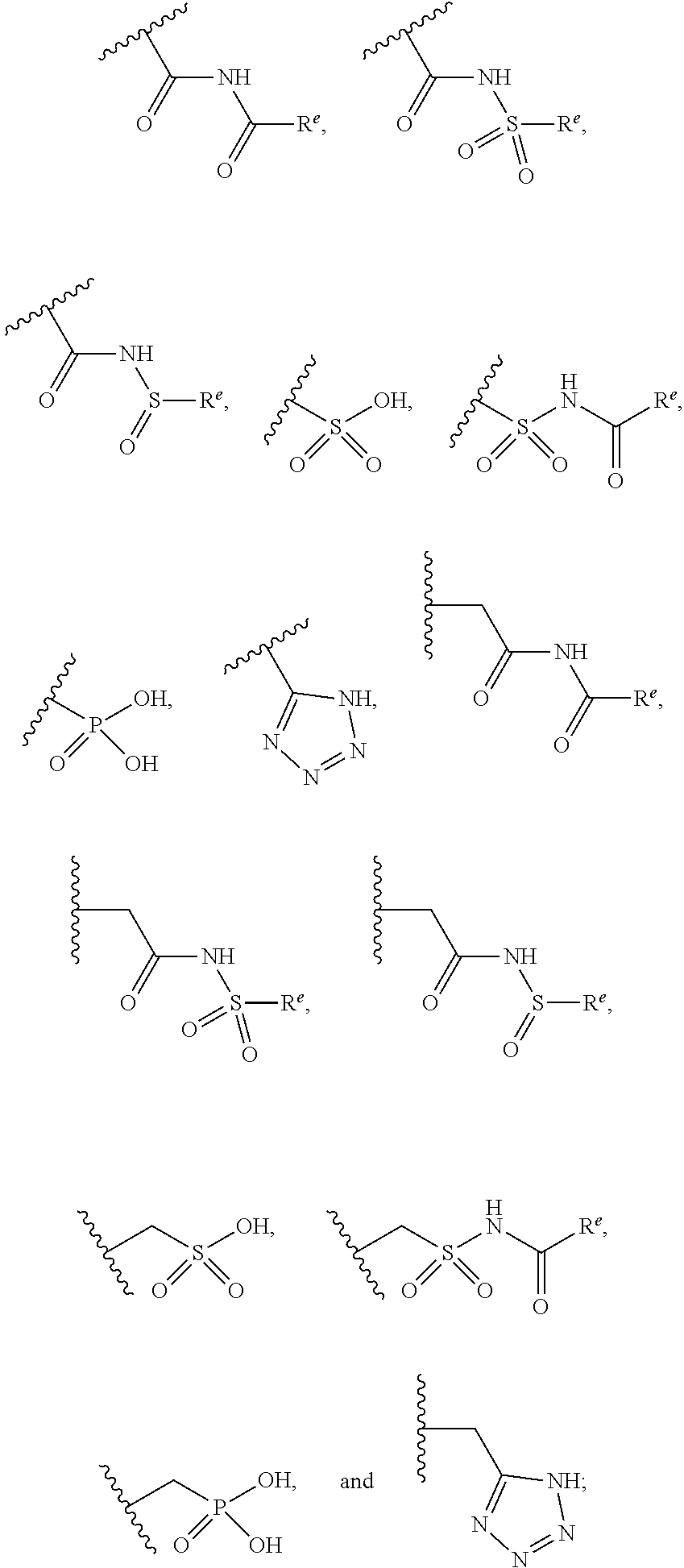
The present invention provides compounds of Formula (I) or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein X1, X2, X3, and X4 are each independently CR6 or N; provided that no more than two of X1, X2, X3, or X4 are N; Q2 is N or NR5a; one of Q1 and Q3 is CR5, and the other is N or NR5a; and the dashed circle denotes optional bonds forming an aromatic ring; Y1 is O or NR3; Y2 is —CO—, —SO2—, or —S(O(NH)—; Y3 is O or NR4a; provided that (1) Y1 and Y3 are not both O, and (2) when Y2 is C(O), Y1 is not O; L is a covalent bond or C1-4 alkylene substituted with 0 to 4 R7; R1 is (—CH2)aR9; a is an integer of 0 or 1; R2 is each independently halo, cyano, hydroxyl, amino, C1-6 alkyl, C3-6 cycloalkyl, C4-6 heterocyclyl, alkylamino, haloalkyi, hydroxyalkyi, aminoalkyi, alkoxy, alkoxyalkyl, haloalkoxyalkyl, or haloalkoxy; n is an integer of 0, 1, or 2; R3 and R4a are independently hydrogen, C1-6 alkyl, haloalkyi, hydroxyalkyi, aminoalkyi, alkoxyalkyl, haloalkoxyalkyl, alkoxy, or haloalkoxy; R4 is C1-10 alkyl, C1-10 haloalkyi, C1-10 deuterated alkyl, C1-10 alkenyl, C3-8 cycloalkyl, 6 to 10-membered aryl, 3 to 8-membered heterocyclyl, —(Ci-6 alkylene)-(C3-8 cycloalkyl), —(C1-6 alkylene)-(6 to 10-membered aryl), —(C1-6 alkylene)-(3 to 8-membered heterocyclyl), or —(C1-6 alkylene)-(5 to 6-membered heteroaryl); wherein each of the alkyl, alkylene, alkenyl, cycloalkyl, aryl, heterocyclyl, and heteroaryl, by itself or as part of other moiety, is independently substituted with 0 to 3 R; or alternatively, R3 and R4, taken together with the N and 0 atoms which they are attached, form a 4 to 9-membered heterocyclic ring moiety which is substituted with 0 to 3 R8; or alternatively, (R3 and R5a) or (R3 and R5), taken together with the atoms to which they are attached to, form a 5 to 8-membered heterocyclic ring moiety which is substituted with 0 to 3 R8; R5a is hydrogen, C1-6 alkyl, alkylamino, haloalkyl, hydroxyalkyl, aminoalkyl, alkoxyalkyl, haloalkoxyalkyl, alkoxy, or haloalkoxy; R5 and R6 are each independently hydrogen, halo, cyano, hydroxyl, amino, alkyl, alkylamino, haloalkyl, hydroxyalkyl, aminoalkyl, alkoxyalkyl, haloalkoxyalkyl, alkoxy, or haloalkoxy; R7 is halo, oxo, cyano, hydroxyl, amino, C1-6 alkyl, C3-6 cycloalkyl, C4-6 heterocyclyl, alkylamino, haloalkyl, hydroxyalkyl, aminoalkyl, alkoxyalkyl, haloalkoxyalkyl, alkoxy, or haloalkoxy; R8 are each independently deuterium, halo, hydroxyl, amino, cyano, C1-6 alkyl, C1-6 deuterated alkyl, C2-6 alkenyl, C2-6 alkynyl, alkylamino, haloalkyl, hydroxyalkyl, aminoalkyl, alkoxyalkyl, haloalkoxyalkyl, alkoxy, haloalkoxy, phenyl, or 5 to 6-membered heteroaryl; or alternatively, two R8, taken together with the atom(s) to which they are attached, form a 3 to 6-membered carbocyclic ring or a 3 to 6-membered heterocyclic ring each of which is independently substituted with 0 to 3 R12; R9 is selected from —CN, —C(O)OR10, —C(O)NR11aR11b—, —CO—NH—CO—Re, —CO—NH—SO2—Re, —CO—NH—SO—Re, —SO2—OH, —SO2—NH—CO—Re, —P(O)(OH)2, tetrazol-5-yl, —CH2—CO—NH—CO—Re, —CH2—CO—NH—SO2—Re, —CH2—CO—NH—SO—Re, —CH2—SO2—OH, —CH2—SO2—NH—CO—Re, —CH2—P(O)(OH)2, tetrazol-5-ylmethylene; Re is C1-6 alkyl, C3-6 cycloalkyl, haloalkyl, hydroxyalkyi, aminoalkyi, alkoxyalkyi, or haloalkoxyalkyi; R10 is hydrogen or C1-10 alkyl; and R11a and R11b are each independently hydrogen, C1-6 alkyl, C3-6 cycloalkyl, C4-6 heterocyclyl, alkylamino, haloalkyi, hydroxyalkyi, aminoalkyi, alkoxyalkyi, haloalkoxyalkyi, alkoxy, or haloalkoxy; and R12 is halo, cyano, hydroxyl, amino, C1-6 alkyl, alkylamino, haloalkyi, hydroxyalkyi, aminoalkyi, alkoxyalkyi, haloalkoxyalkyi, alkoxy, haloalkoxy, phenyl, or 5 to 6-membered heteroaryl. These compounds are selective LPA receptor inhibitors.
Owner:BRISTOL MYERS SQUIBB CO
Cyclohexyl acid isoxazole azines as lpa antagonists
ActiveUS20200317656A1Organic active ingredientsOrganic chemistryLPA ReceptorsPharmaceutical medicine
Owner:BRISTOL MYERS SQUIBB CO
Salts of Halogen Substituted Heterocyclic Compounds
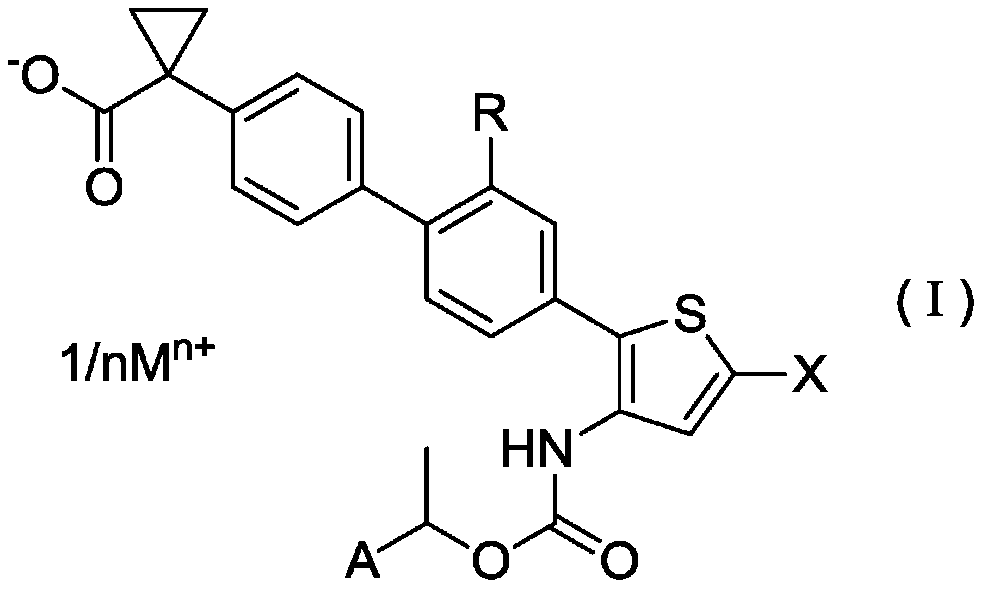
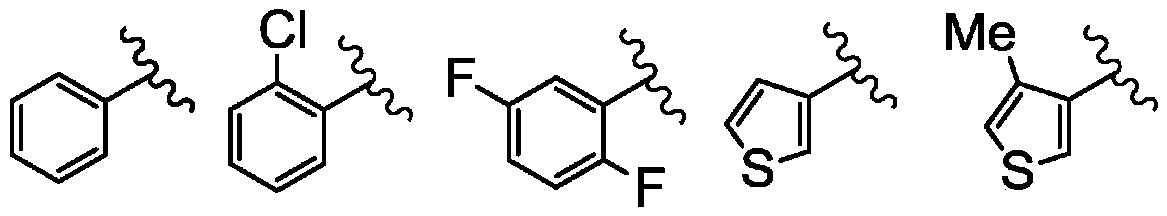
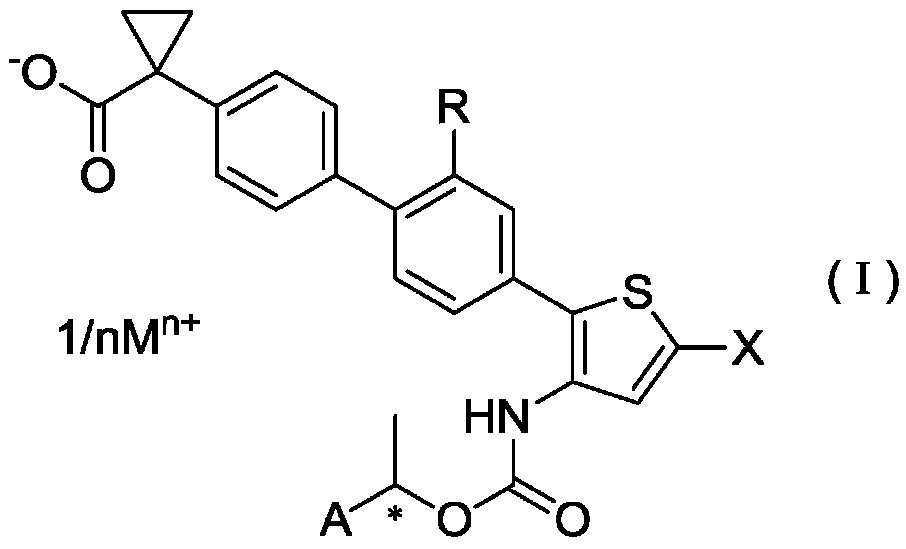
ActiveCN106458964BPotent LPA receptor antagonismOrganic active ingredientsNervous disorderHydrogenHydrogen atom
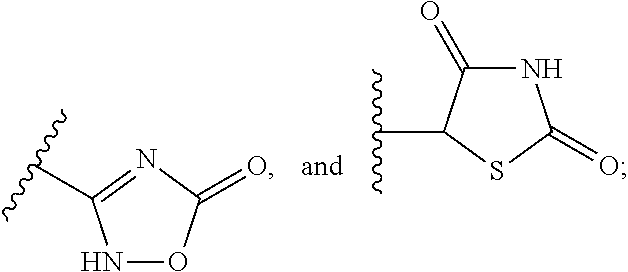
To provide a salt of a novel α-position halogen-substituted thiophene compound which has potent LPA receptor antagonistic activity and is useful as a drug. A salt shown in general formula (I), wherein, R is a hydrogen atom or a methoxyl group; X is a halogen atom; A is a group selected from the group consisting of the following groups (AA); M is Alkali metal or alkaline earth metal; n represents 1 when M is an alkali metal, and 2 when M is an alkaline earth metal.
Owner:UBE IND LTD
Cyclohexyl acid pyrazole azines as lpa antagonists
ActiveUS20210087178A1Organic active ingredientsOrganic chemistryLPA ReceptorsPharmaceutical medicine
Owner:BRISTOL MYERS SQUIBB CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com