LPA receptor agonists and antagonists and methods of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
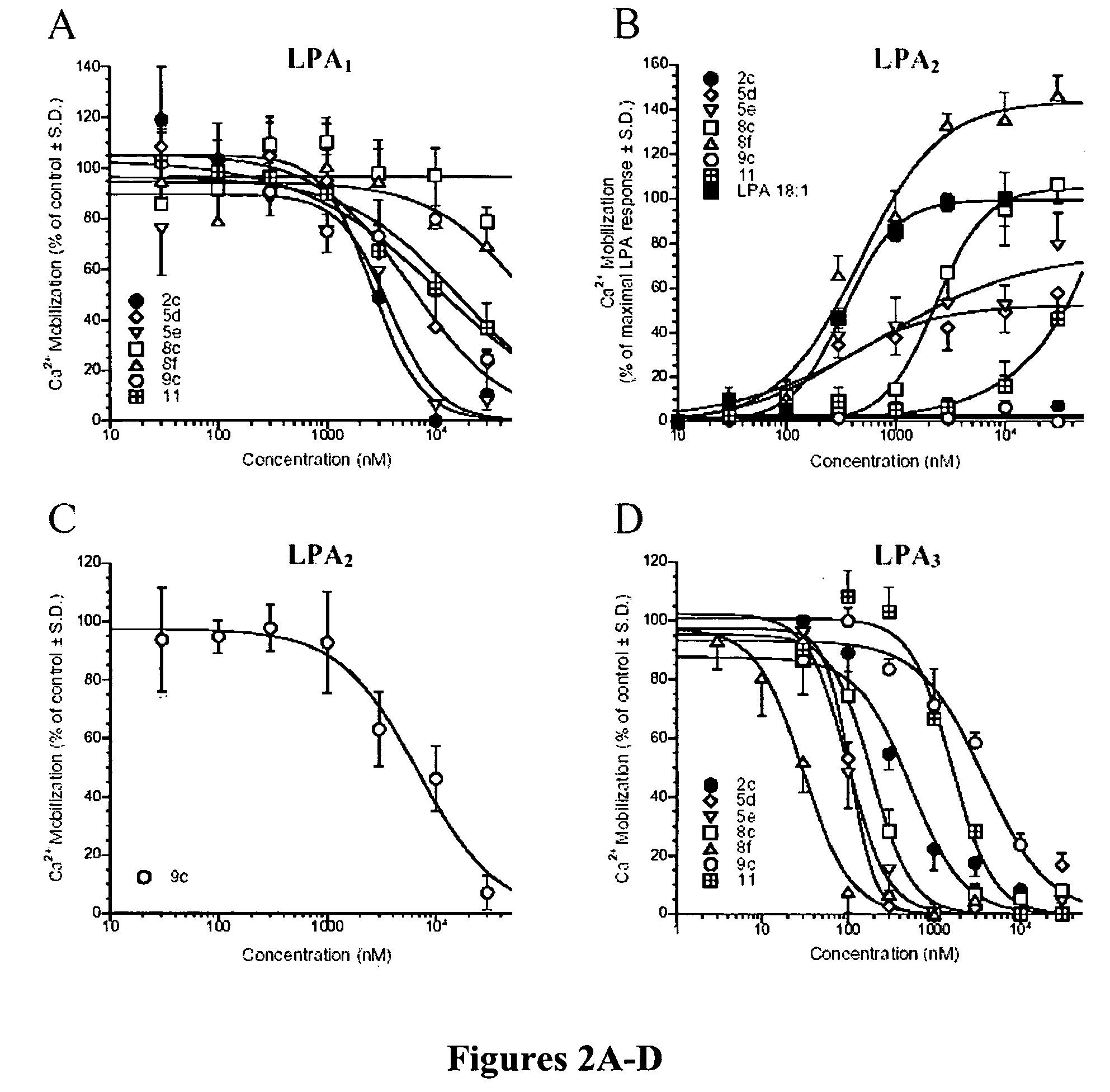
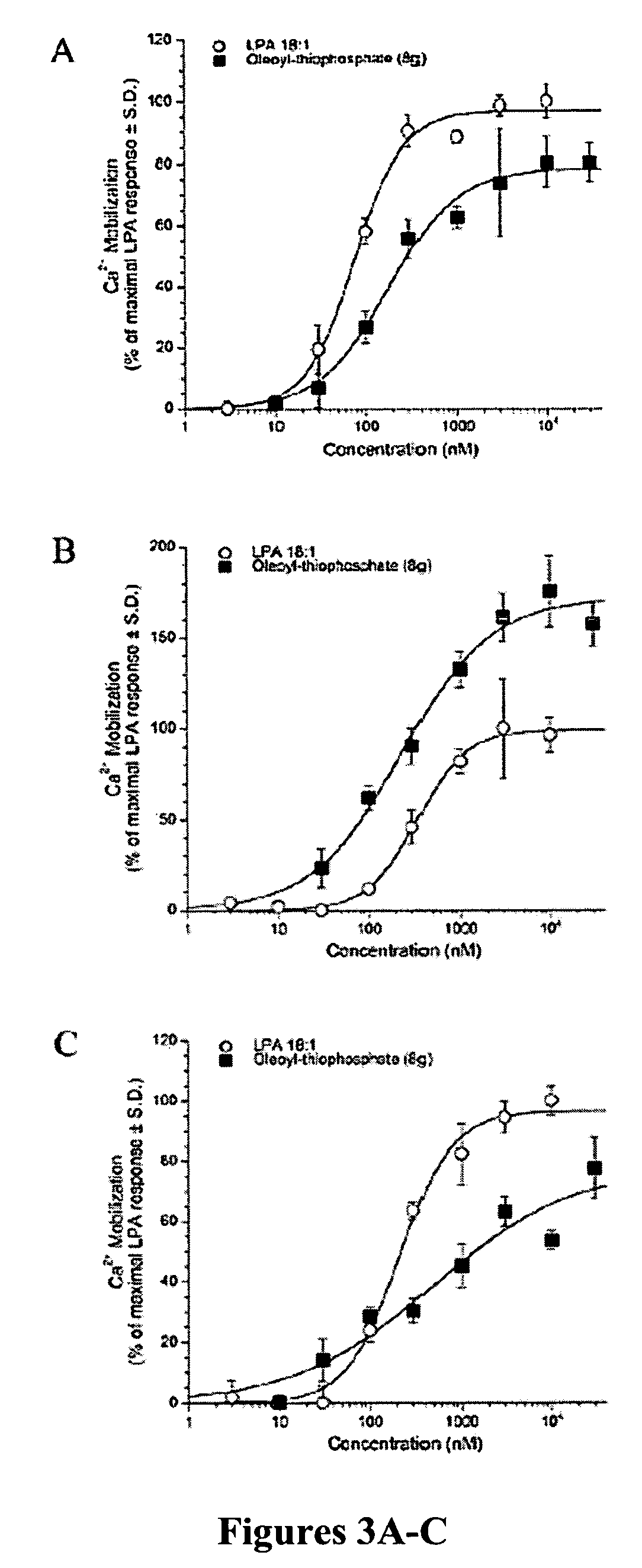
Examples
example 1
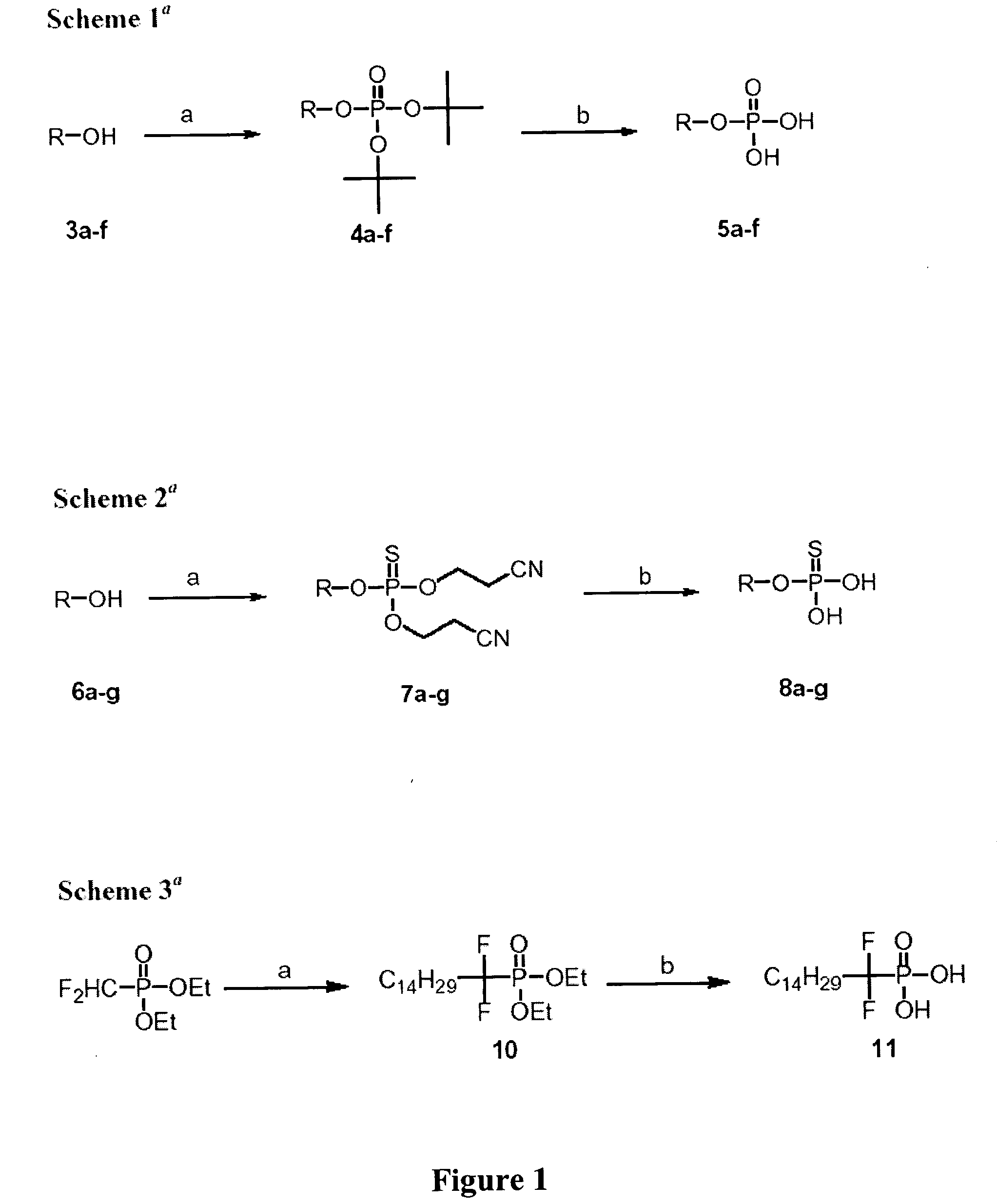
Synthesis of Phosphoric Acid di-tert-butyl Ester Alkenyl Esters (4a-f)
[0172] Commercially available unsaturated fatty alcohols (3a-f) were used as starting materials. To a stirred solution of alcohol (2.5 mmol) and di-tert-butyl-N,N-diisopropyl phosphoramidite (1.51 g, 4 mmol) in methylene chloride (60 mL) was added 1H-tetrazole (578 mg, 8.25 mmol). After 30 minutes of stirring the mixture was cooled to 0° C. and 0.3 mL of 50% hydrogen peroxide was added. The mixture was stirred for 1 h., diluted with methylene chloride (100 mL), washed with 10% sodium metabisulfite (2×50 ml), saturated sodium bicarbonate (2×50 ml), water (50 ml), and brine (50 ml). The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under vacuum. The resulting crude products were purified by silica gel chromatography using hexane / ethyl acetate (7:3) to elute the desired products, di-t-Boc protected fatty alcohol phosphates (4a-f).
[0173] Phosphoric acid di-tert-butyl ester dec-9-e...
example 2
Synthesis of Phosphoric Acid Mono Alkenyl Esters (5a-f)
[0179] The Boc-protected FAPs (4a-f) were deprotected with TFA to yield the corresponding unsaturated FAPs (5a-f). To a solution of 100 mg of 1a-6a in methylene chloride (20 mL), trifluroacetic acid (0.3 mL) was added. The mixture was allowed to stir for 4 h., and TLC showed the completion of the reaction. Solvents were evaporated; the residue was washed with methylene chloride (2×20 mL), and concentrated under vacuum to yield the desired phosphoric acid mono alkenyl esters as colorless oils.
[0180] Phosphoric acid monodec-9-enyl ester (5a): Isolated as an oil (85%). 1H NMR (MeOH-d4): δ 5.74 (m, 1H), 4.88 (m, 2H), 3.90 (q, J=6.6 Hz, 2H), 2.01 (q, J=6.9 Hz, 2H), 1.61 (quintet, 2H), 1.28 (br s, 10H); 31PNMR (MeOH-d4): δ 17.84; MS: [M−H]− at m / z 235.2. Anal. (C10H21O4P.0.1H2O)C, H.
[0181] Phosphoric acid monodec-4-enyl ester (5b): Isolated as an oil (78%). 1H NMR (MeOH-d4): δ 5.31 (m, 2H), 3.84 (q, J=6.8 Hz, 2H), 2.05 (q, J=7.0 Hz...
example 3
Synthesis of Thiophosphoric Acid O,O′-bis-(2-cyano-ethyl) Ester O″-alkyl / alkenyl Esters (7a-g)
[0186] Commercially available saturated or unsaturated fatty alcohols (6a-g) were used as starting materials. A solution of alcohol (2.0 mmol), bis-(2-cyanoethyl)-N,N-diisopropyl phosphoramidite (1.085 g, 4 mmol) and 1H-tetrazole (420 mg, 6 mmol) was stirred for 30 minutes at room temperature, followed by the addition of elemental sulfur (200 mg) and the mixture was refluxed for 2 h. The reaction mixture was cooled to room temperature and solvents were evaporated under vacuum. Addition of ethyl acetate (30 mL) precipitated excess sulfur, which was filtered out, and the solvent was evaporated to give the crude mixture. The mixture was purified by flash chromatography to give the desired products as colorless oils.
[0187] Thiophosphoric acid O,O′-bis-(2-cyano-ethyl) ester O″-decyl ester (7a): Isolated as colorless oil (72% yield). 1H NMR (CDCl3): δ 4.21-4.35 (m, 4H), 4.12 (m, 2H), 2.8 (t, J=...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acidity | aaaaa | aaaaa |
| Cell proliferation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com