Application of serine in preparing medication for treating cerebral ischemia
A technology of serine and cerebral ischemia, applied in the direction of drug combination, cardiovascular system diseases, etc., can solve the problems of no application of cerebral ischemia, and achieve the best effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
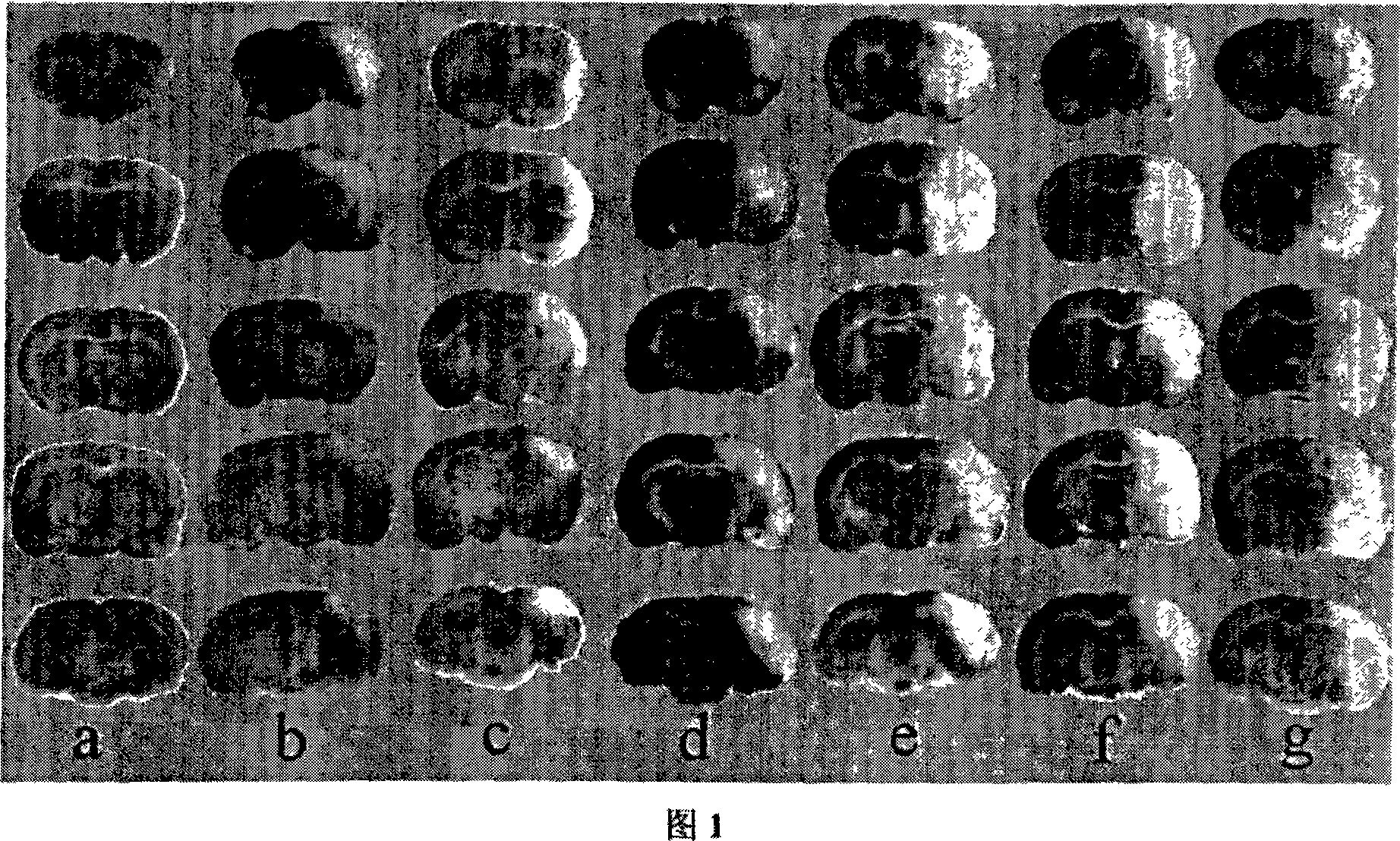
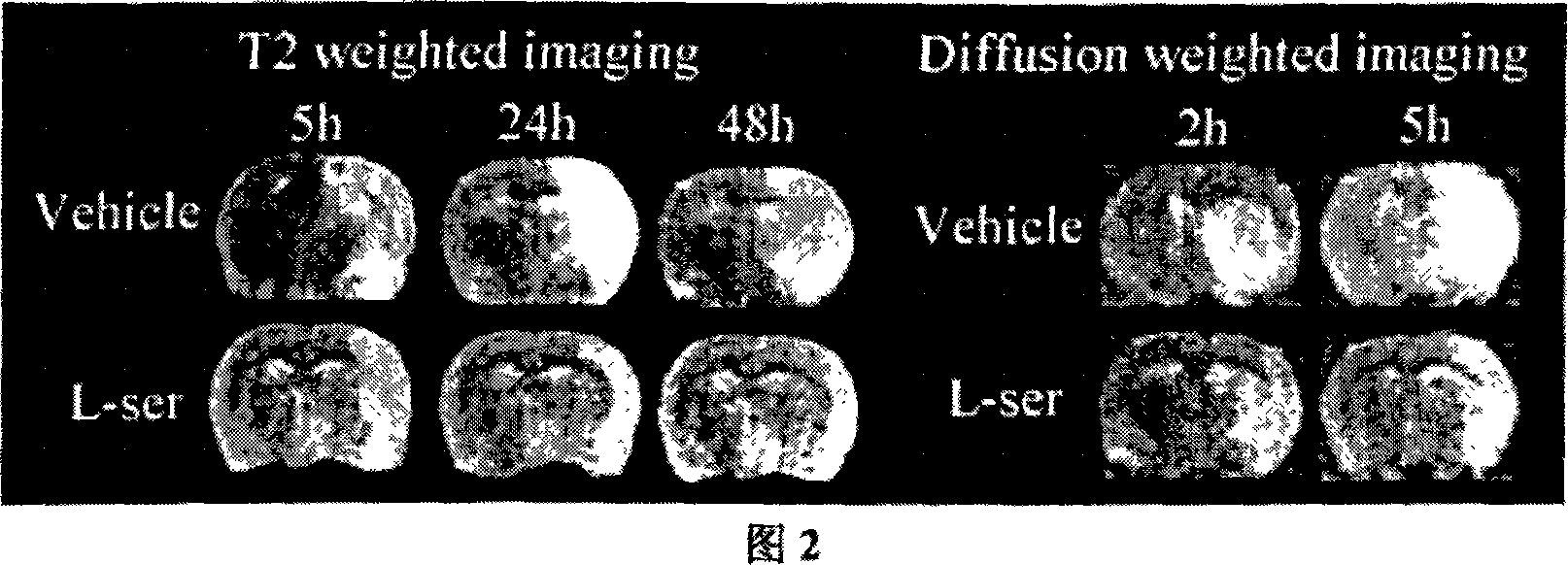
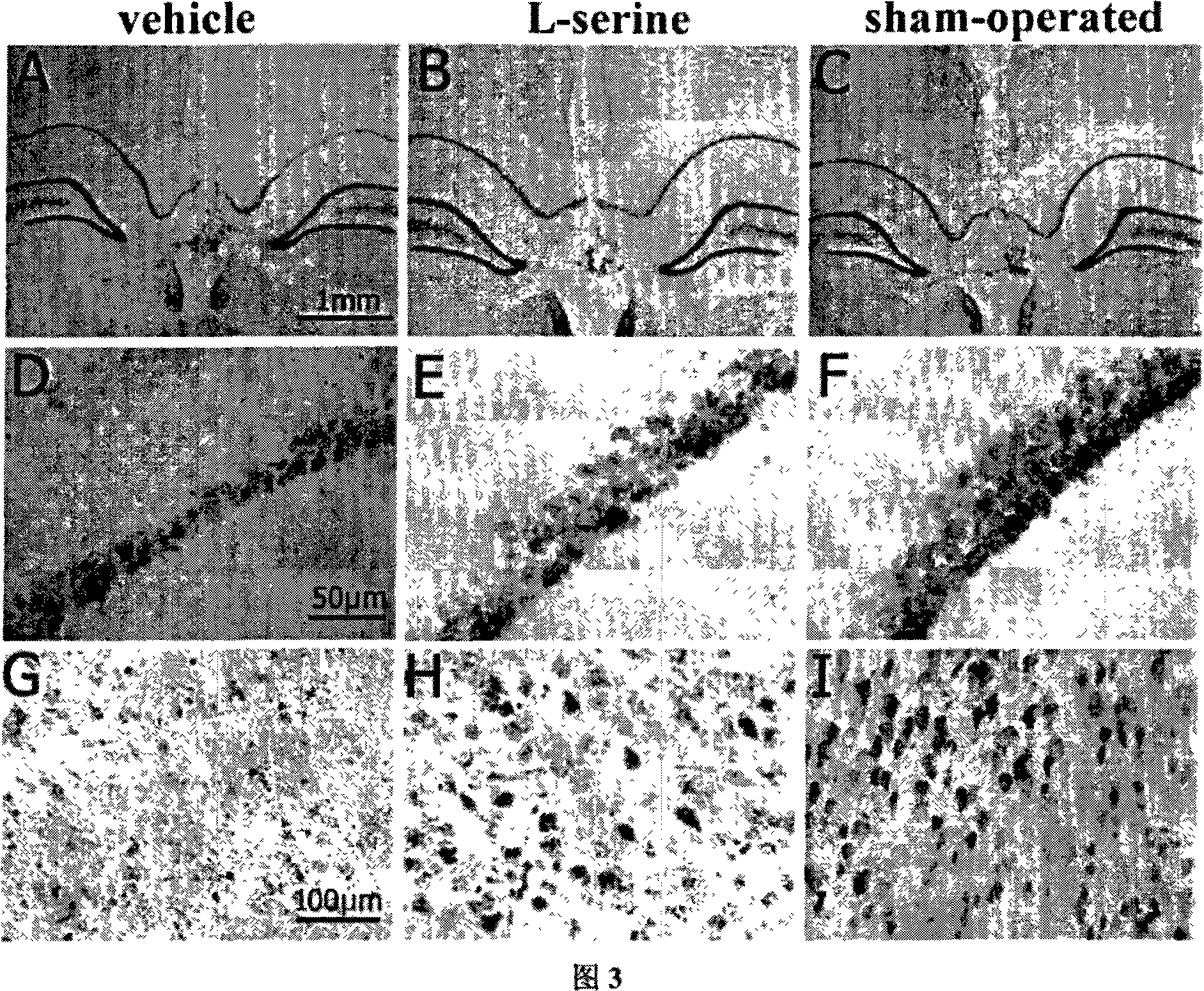
[0033] An application of L-serine in the preparation of medicine for treating cerebral ischemia.
[0034] Specific positive test:
[0035] 1. Experimental materials and methods
[0036] 1 material
[0037] 1.1 Experimental animals: healthy adult male Sprague-Dawley (SD) rats, clean grade, weighing 240-260g, provided by the Experimental Animal Center of Nantong University.
[0038] 1.2 Main reagents and solutions: Taurine (Taurine) powder was purchased from Sigma-Aldrich; Glycine: Sinopharm Chemical Reagent Co., Ltd.; L-serine: Sinopharm (Group) Shanghai Chemical Reagent Company; β-Alanine: Sigma Company (U.S.); L-alpha-alanine: China Pharmaceutical (Group) Shanghai Chemical Reagent Company; red tetrazolium (2,3,5-triphenyltetrazolium, TTC) powder was purchased from Shanghai Lingjin Fine Chemical Co., Ltd.; anti-β -actin monoclonal antibody was purchased from Sigma; Cleaved Caspase-3 (Asp175) rabbit anti-mouse monoclonal antibody was purchased from CellSignaling; the seconda...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com