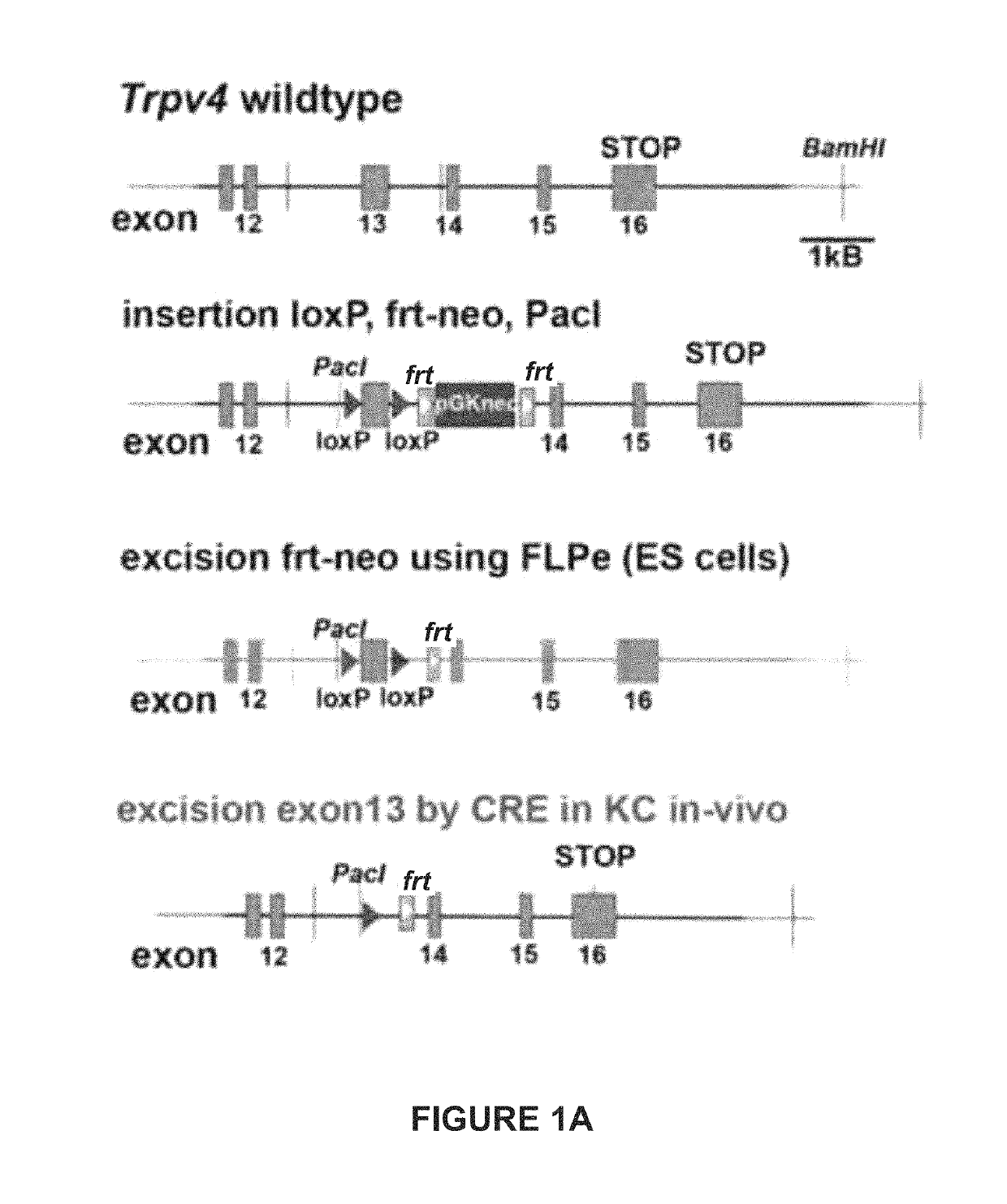
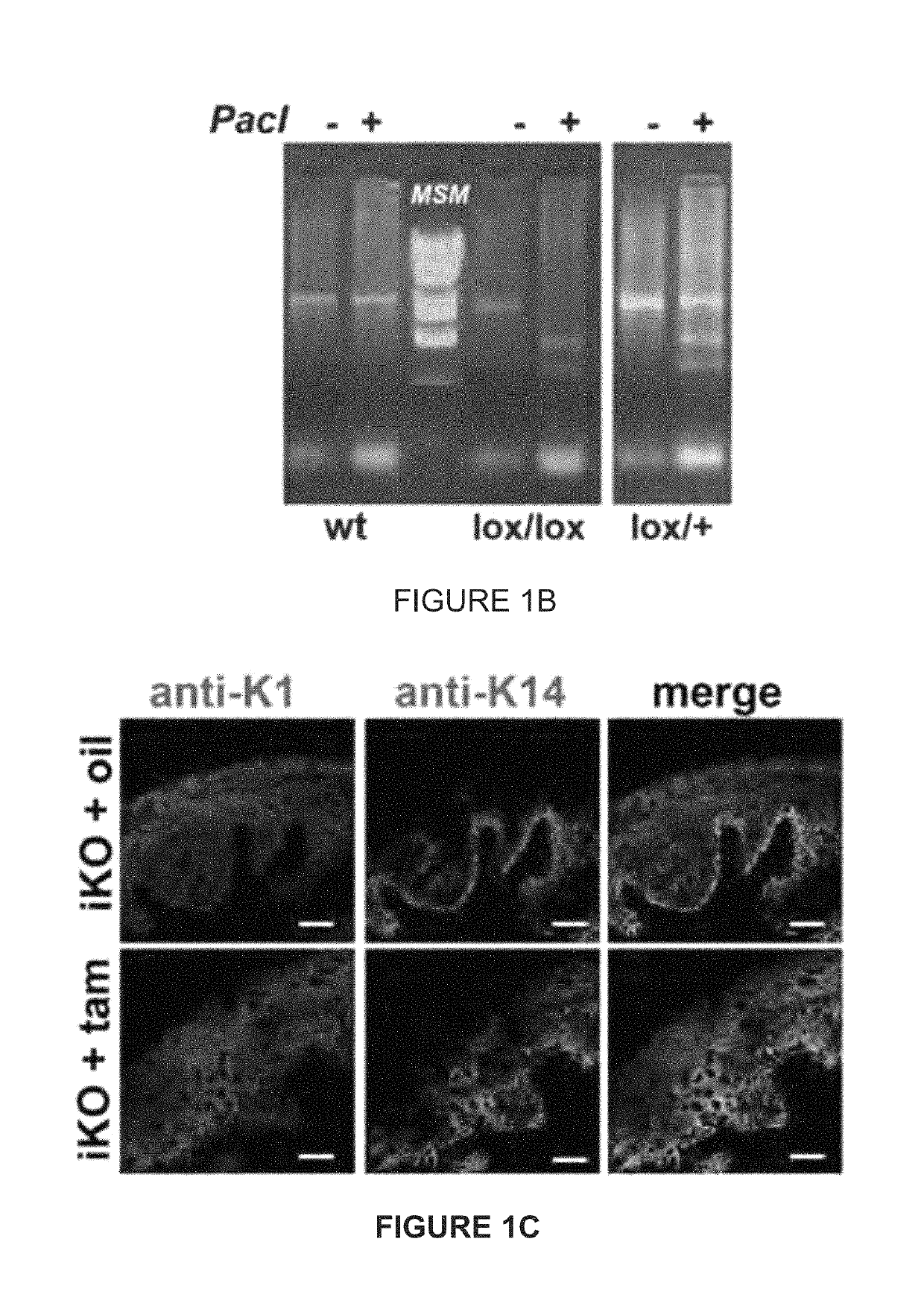
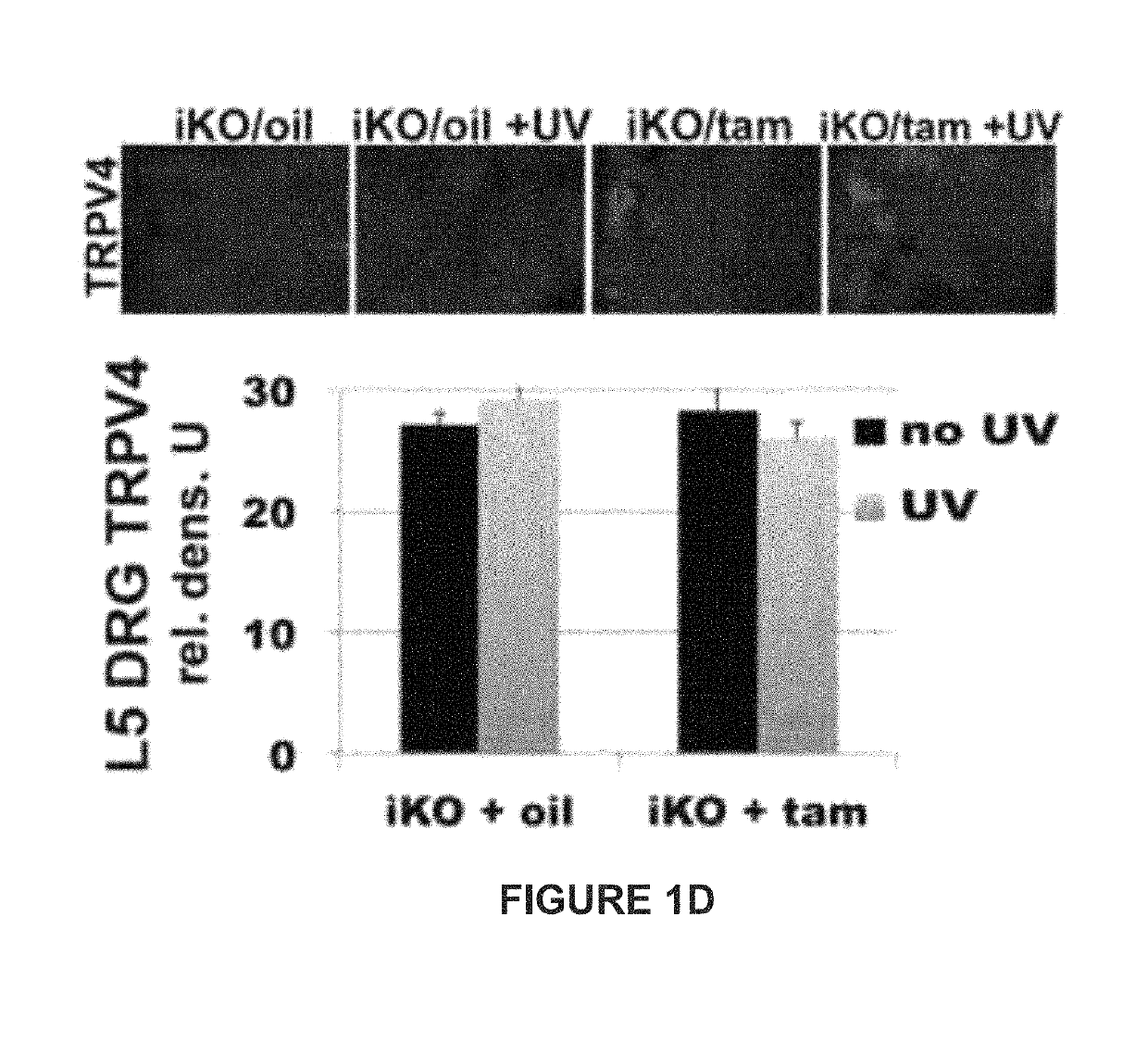
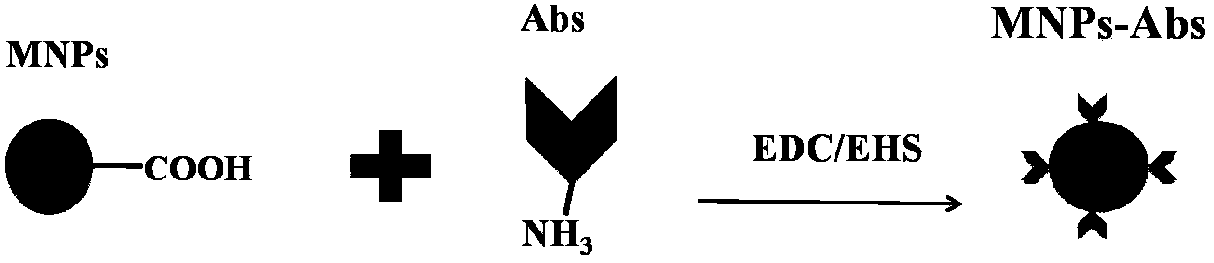Patents
Literature
58 results about "TRPV4" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transient receptor potential cation channel subfamily V member 4 is an ion channel protein that in humans is encoded by the TRPV4 gene. The TRPV4 gene encodes TRPV4, initially named "vanilloid-receptor related osmotically activated channel" (VR-OAC) and "OSM9-like transient receptor potential channel, member 4 (OTRPC4)", a member of the vanilloid subfamily in the transient receptor potential (TRP) superfamily of ion channels. The encoded protein is a Ca²⁺-permeable, nonselective cation channel that has been found involved in multiple physiologic functions, dysfunctions and also disease. It functions in the regulation of systemic osmotic pressure by the brain, in vascular function, in liver, intestinal, renal and bladder function, in skin barrier function and response of the skin to ultraviolet-B radiation, in growth and structural integrity of the skeleton, in function of joints, in airway- and lung function, in retinal and inner ear function, and in pain. The channel is activated by osmotic, mechanical and chemical cues. It also responds to thermal changes (warmth). Channel activation can be sensitized by inflammation and injury.
Trpv4 antagonists
Owner:GLAXO SMITHKLINE LLC
Activation of TRPV4 ion channel by physical stimuli and critical role for TRPV4 in organ-specific inflammation and itch
ActiveUS9290489B2Compounds screening/testingOrganic active ingredientsDiluentDermatological disorders
Provided are TRPV4 inhibitor compounds. Further provided are compositions including a TRPV4 inhibitor compound in combination with a carrier, vehicle, or diluent that is suitable for topical application. The TRPV4 inhibitor compounds and compositions may be used in methods of treating and / or preventing dermatological disorders, reducing skin inflammation, reducing pain, and / or reducing itch in a subject in need thereof.
Owner:DUKE UNIV
Novel Compounds
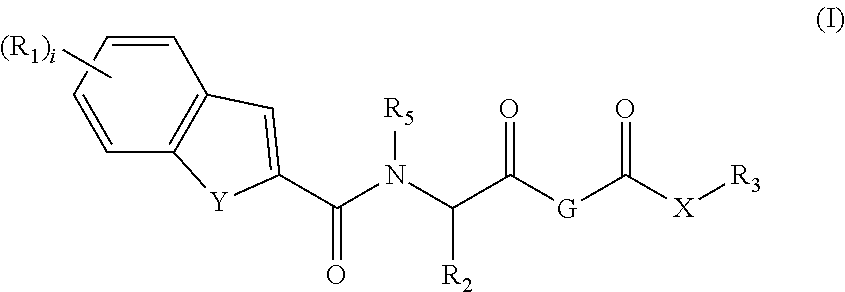
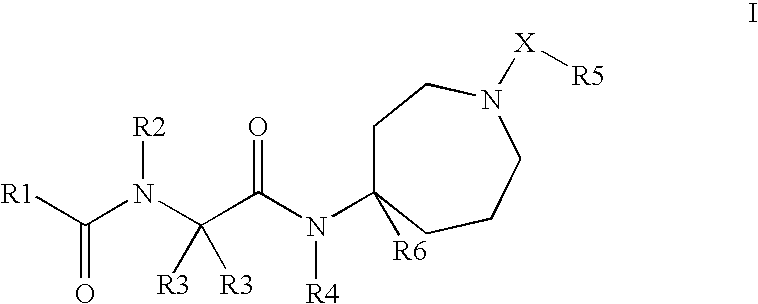
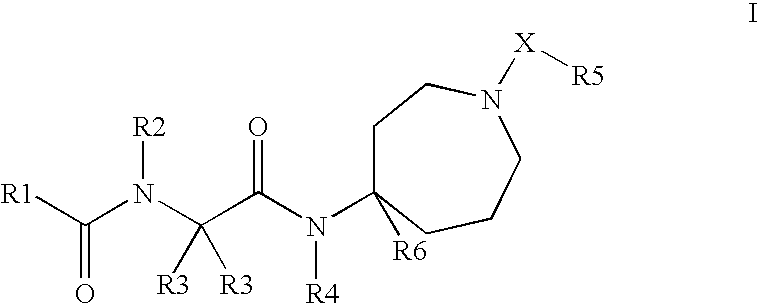
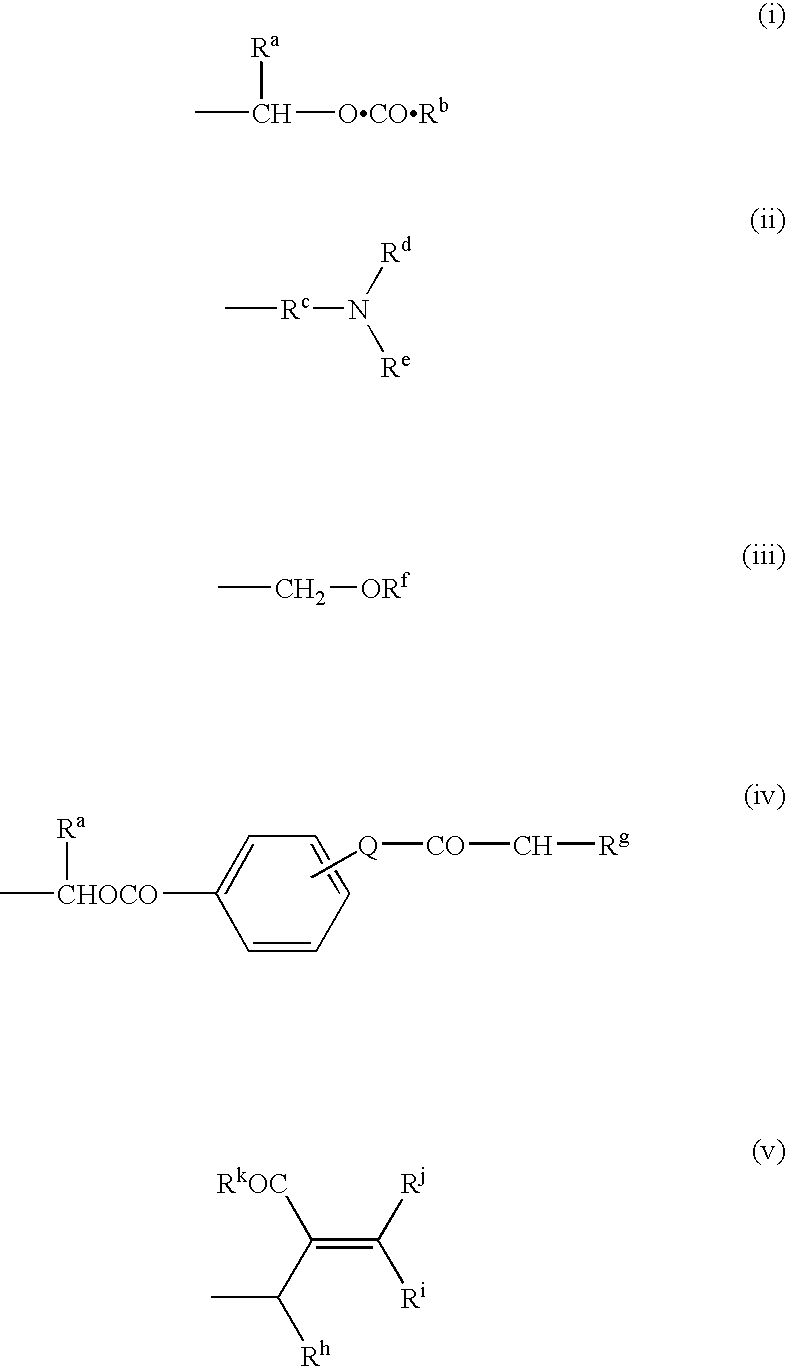
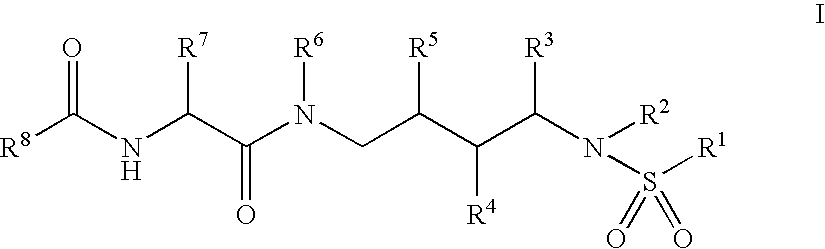
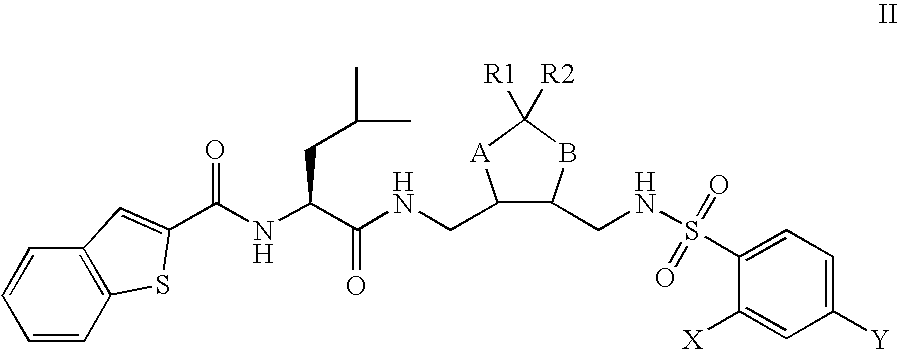
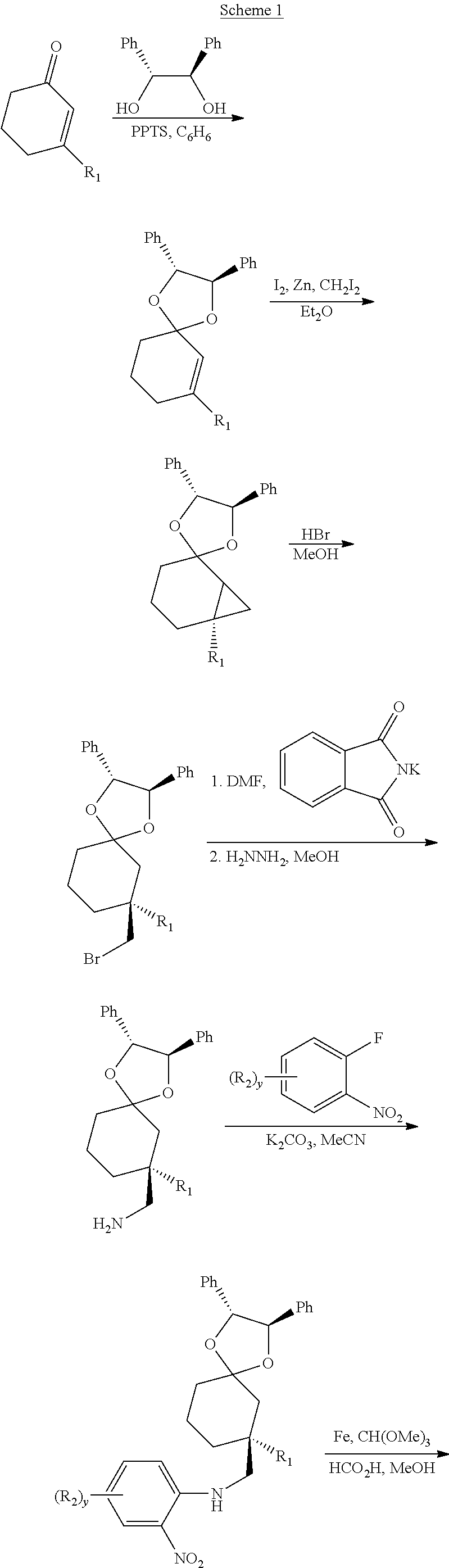
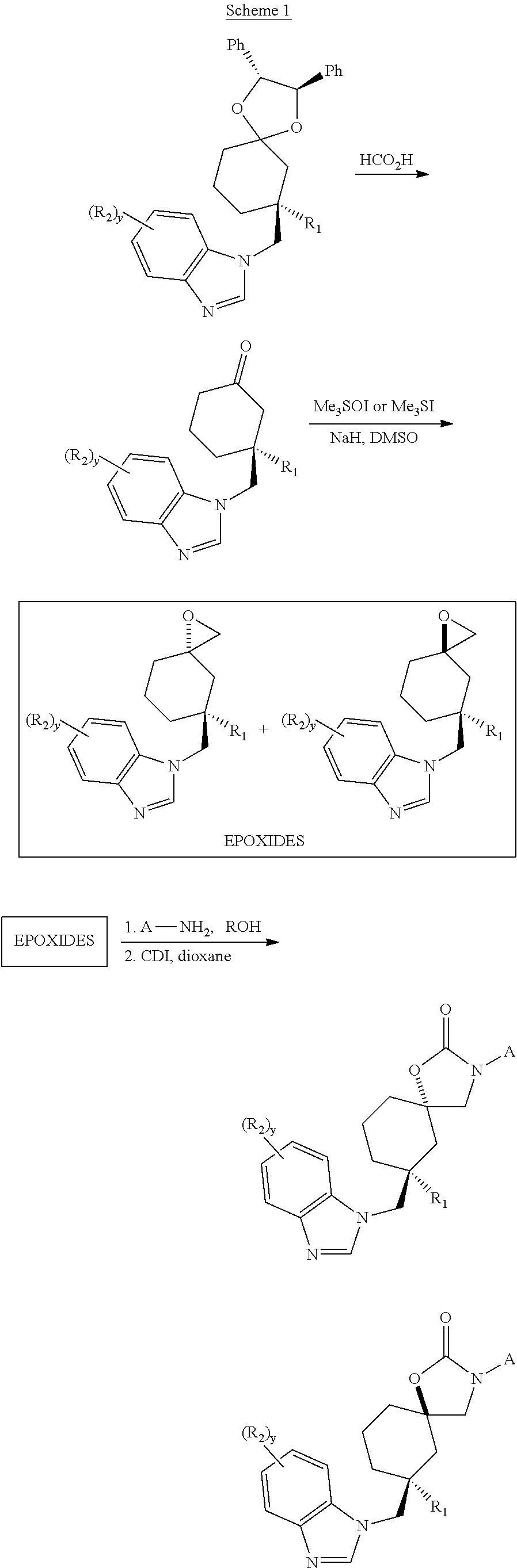
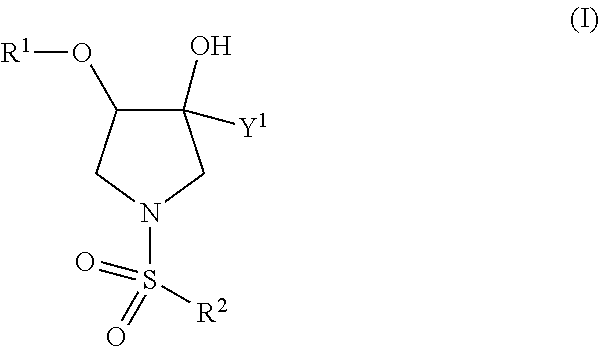
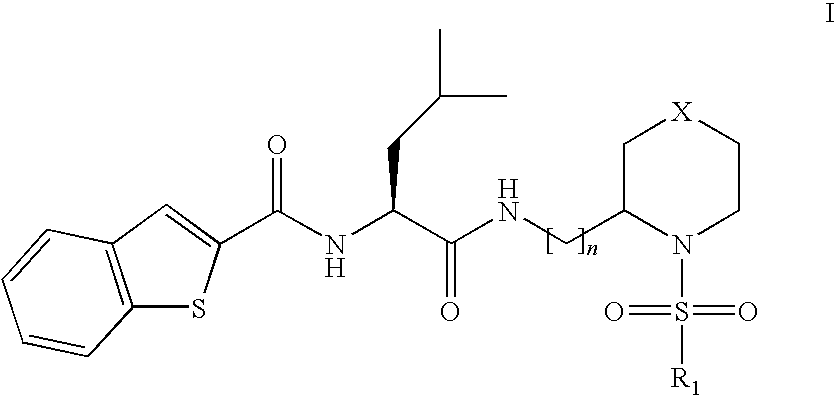
This invention relates to novel compounds useful in the treatment of diseases associated with TRPV4 channel receptor. More specifically, this invention relates to certain substituted amino-azepines, according to Formula I Specifically, the invention is directed to compounds according to Formula I wherein: R1 is optionally substituted C3-7cycloalkyl, optionally substituted C3-7cycloalkenyl, optionally substituted Het-C3-7alkyl, optionally substituted Het-C3-7alkenyl, optionally substituted aryl, optionally substituted heterocycloalkyl, optionally substituted heteroaryl, or optionally substituted indenyl; R2 is H, optionally substituted C1-6alkyl, C3-6cycloalkyl-C0-6alkyl, Ar—C0-6alkyl, or Het-C0-6alkyl; each R3 is independently H, optionally substituted C1-8alkyl, optionally substituted C2-8alkenyl, optionally substituted C2-8alkynyl, Het-C1-6 alkyl, optionally substituted C3-6cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl, or optionally substituted C1-C6 alkoxy; R4 is H, or optionally substituted C1-C4 alkyl; R5 is H, optionally substituted C1-8alkyl, optionally substituted C2-8alkenyl, optionally substituted C2-8alkynyl, optionally substituted C3-6cycloalkyl, optionally substituted heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; R6 is H or C1-6alkyl; and X is SO2, CO, CH2, or CONH, and pharmaceutically acceptable salts, hydrates, solvates and pro-drugs thereof.
Owner:SMITHKLINE BECKMAN CORP
Trpa1 and trpv4 inhibitors and methods of using the same for organ-specific inflammation and itch
Owner:DUKE UNIV
TRPA1 and TRPV4 inhibitors and methods of using the same for organ-specific inflammation and itch
Owner:RGT UNIV OF CALIFORNIA +1
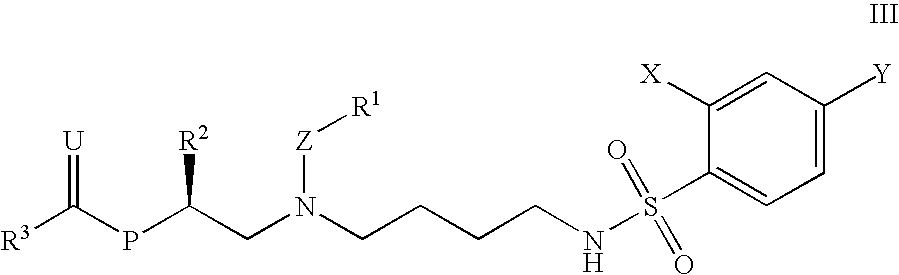
Acyclic 1,4-Diamines and Uses Thereof
This invention relates to novel compounds useful in the treatment of diseases associated with TRPV4 channel receptor. More specifically, this invention relates to certain acyclic diamines, which are agonists of TRPV4 channel receptors.
Owner:SMITHKLINE BECKMAN CORP
Small molecule dual-inhibitors of trpv4 and trpa1 for sanitizing and anesthetizing
ActiveUS20190091206A1Relieve painOrganic active ingredientsAntipyreticDiluentDermatological disorders
Provided are methods of sanitizing a subject, and methods of anesthetizing a subject. Further provided are methods of treating and / or preventing dermatological disorders, reducing skin inflammation, reducing pain, and / or reducing itch in a subject in need thereof. The methods may include administering to the subject an effective amount of a TRPA1 and / or TRPV4 inhibitor. Further provided are compositions including a TRPA1 and / or TRPV4 inhibitor compound in combination with a carrier, vehicle, or diluent that is suitable for topical application.
Owner:DUKE UNIV
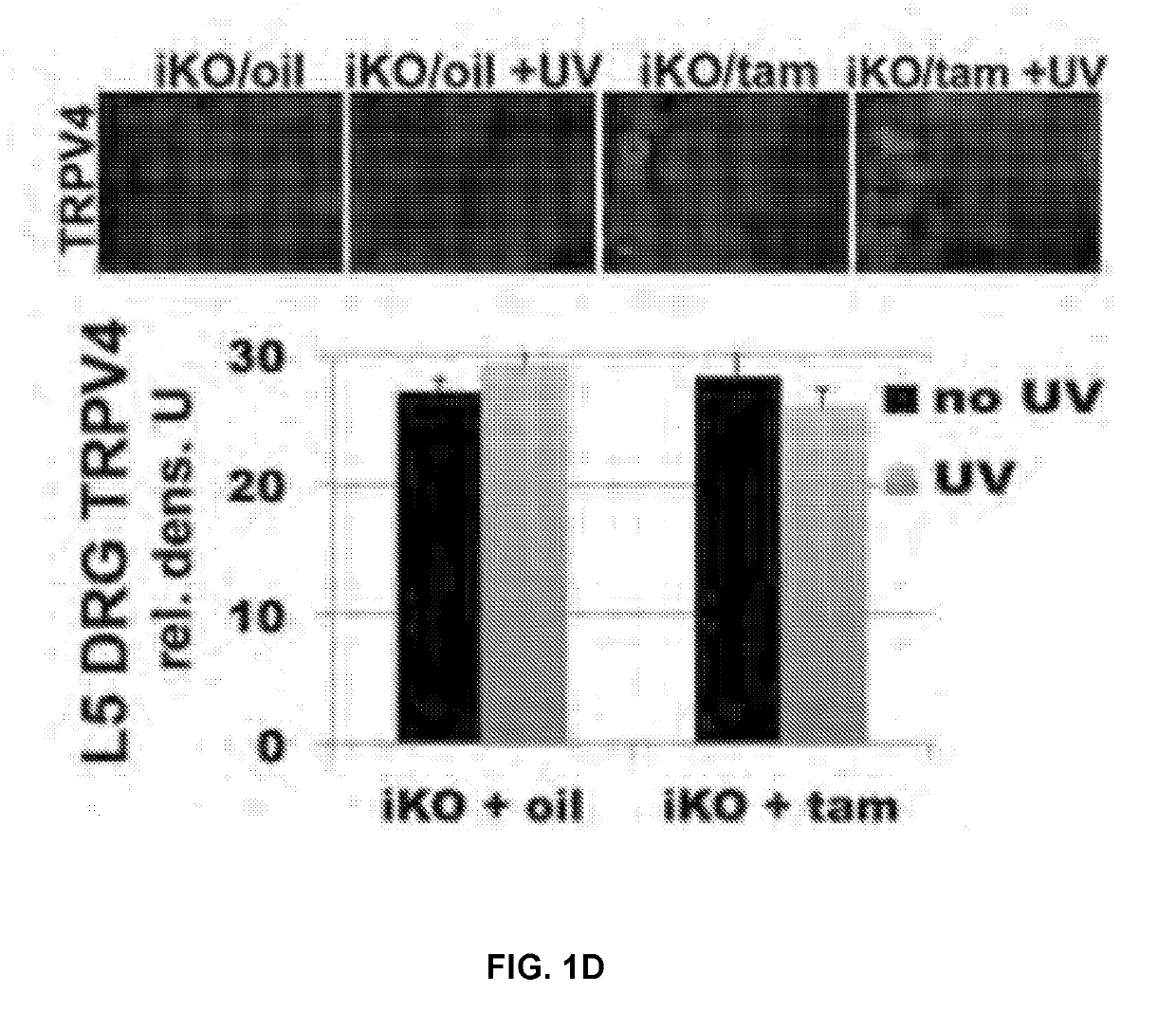
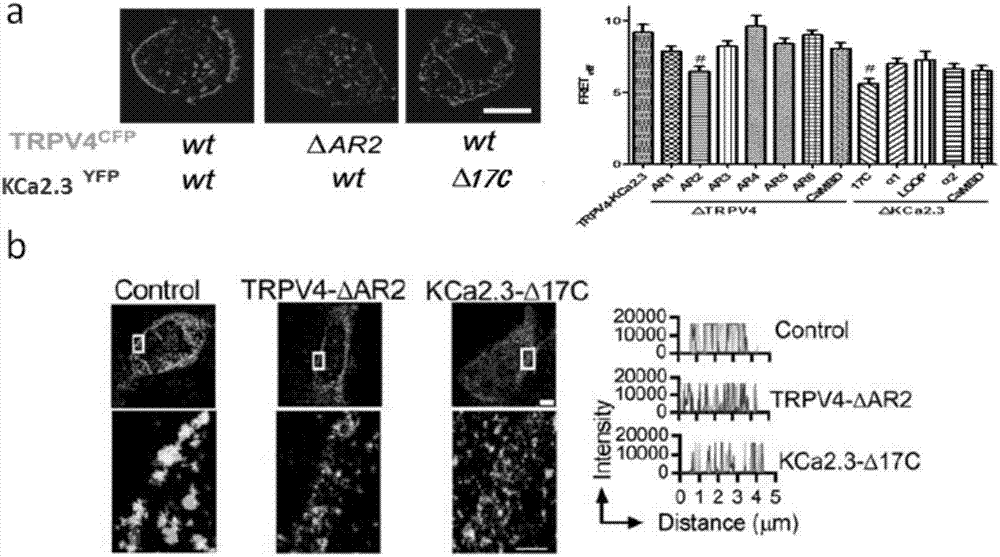
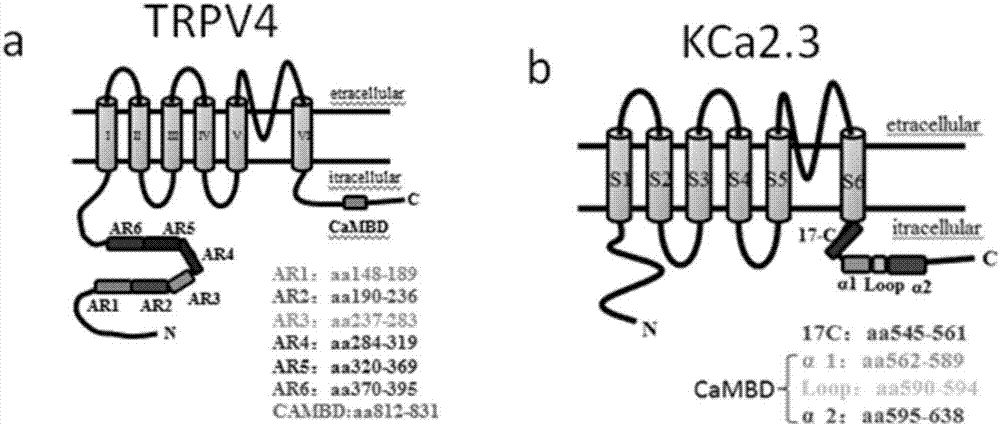
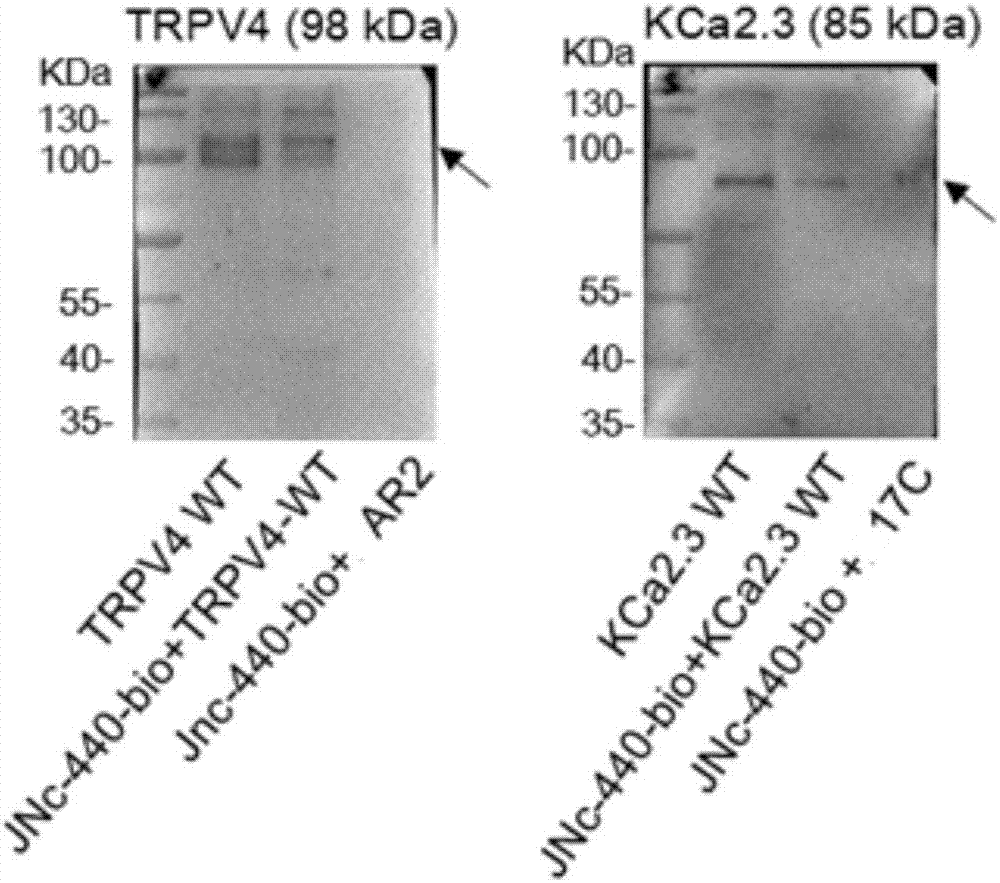
Compound for enhancing coupling degree of complex TRPV4-KCa2.3 and application thereof in resisting hypertension
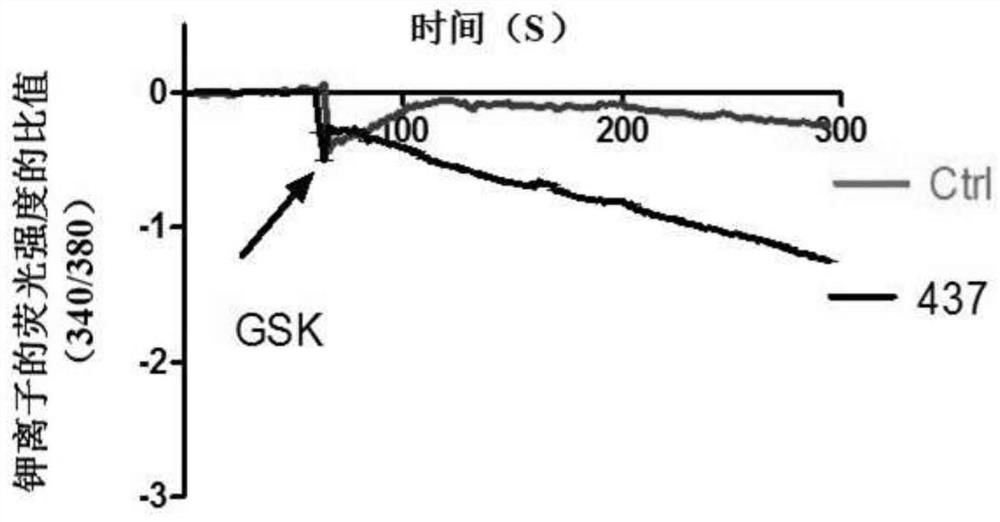
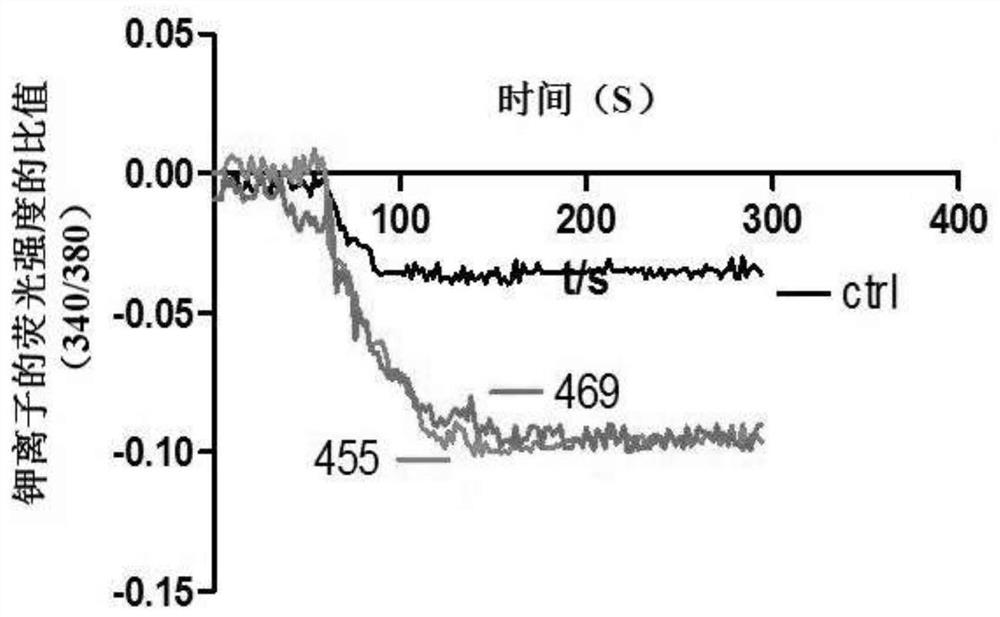
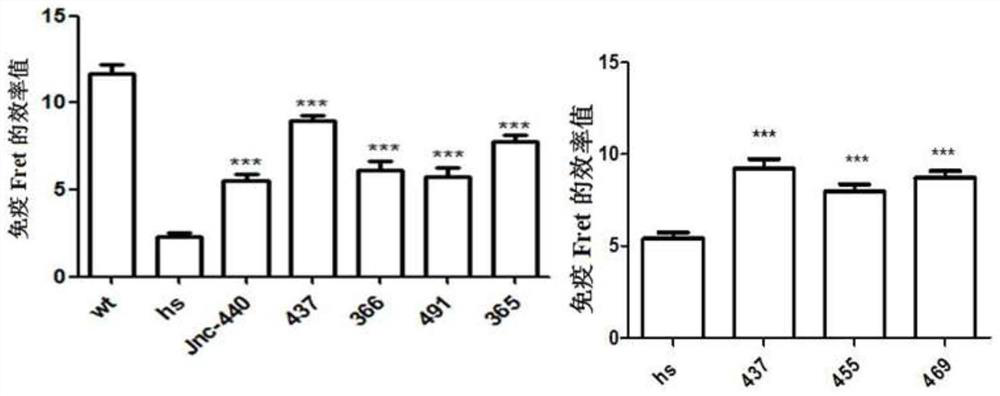
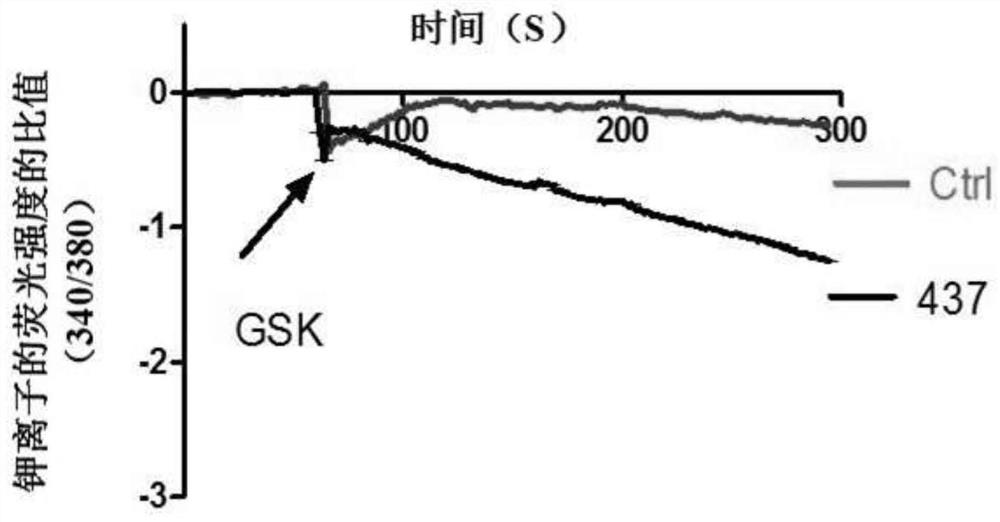
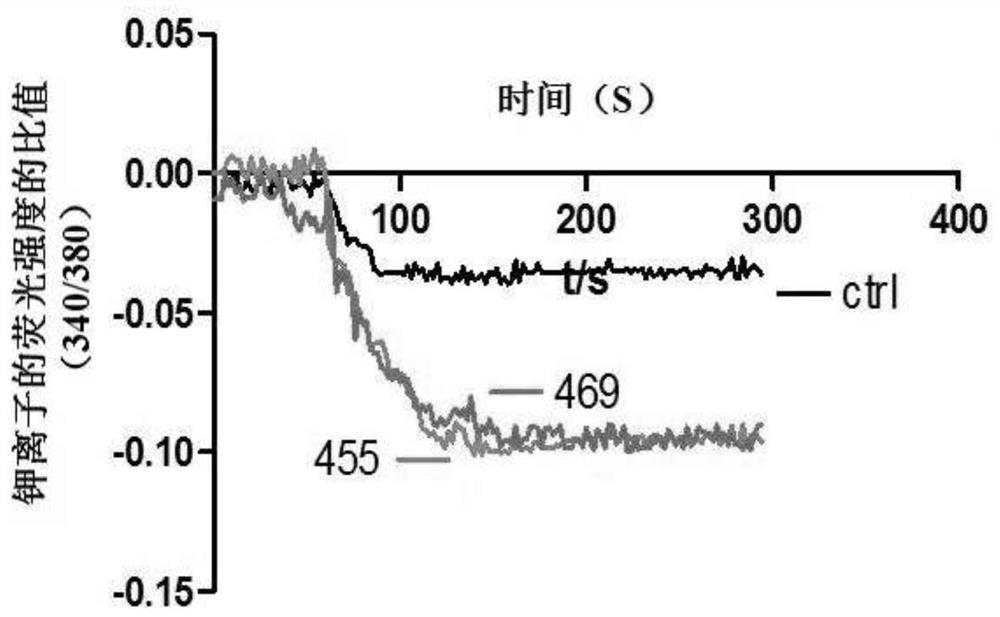
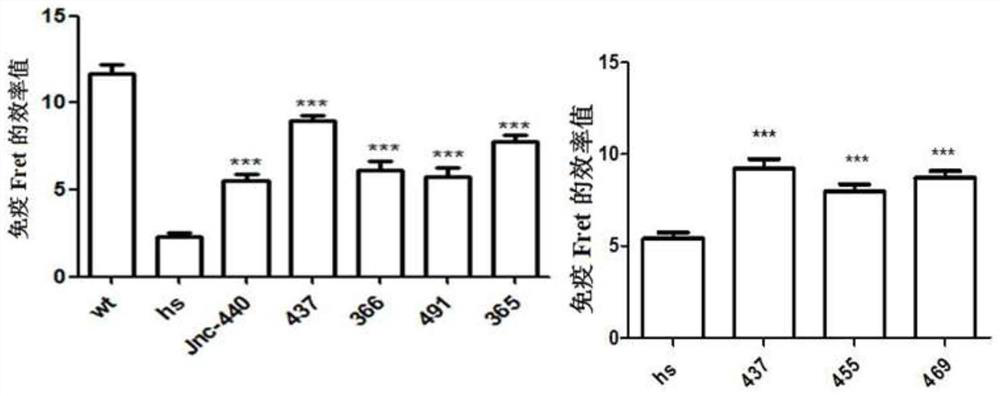
ActiveCN107056715AEnhance spatial couplingSuppress high blood pressureOrganic active ingredientsOrganic chemistryChemical compoundCoupling
The invention provides a compound for enhancing space coupling degree of an endothelial cell ion channel complex TRPV4-KCa2.3 and application thereof in resisting hypertension. The compound has the advantages that by finding the structure domain of interaction sites of the endothelial cell ion channel complex TRPV4-KCa2.3, the specific compound which can simultaneously act on the two action sites is prepared; the space coupling degree of the complex TRPV4-KCa2.3 can be enhanced by the compound, and the important meaning is realized for the research and development of hypertension drugs.
Owner:JIANGNAN UNIV
Methods for generating functional hematopoietic stem cells
Methods for preparing populations of hematopoietic stem cells (HSCs), e.g., autologous and / or allogenic HSCs, using mechanical stretching or transient receptor potential cation channel-subfamily vanilloid member 4 (Trpv4) agonists, and methods of use of the HSCs in transplantation are provided. Specifically, the methods comprising providing population cells comprising hemogenic endothelial (HE) cells, contacting the HE cells with an amount of an agonist of Trpv4; and / or subjecting the cells to cyclic 2-dimensional stretching, for a time and under conditions sufficient to stimulating endothelial-to-HSC transition. Further disclosed are methods of treating malignant or non-malignant hematologic, metabolic, or immune disorders comprising administering to a subject a therapeutically effectiveamount of HSCs obtained by the methods.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Trpv4 antagonists
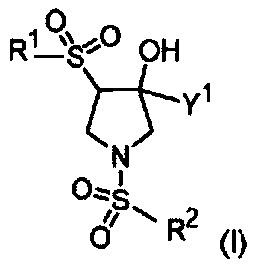
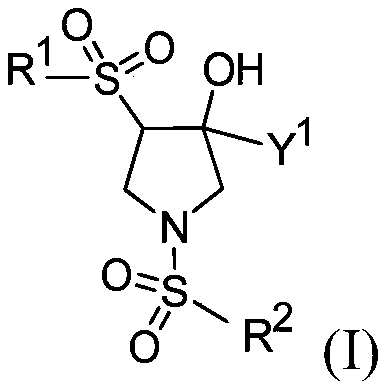
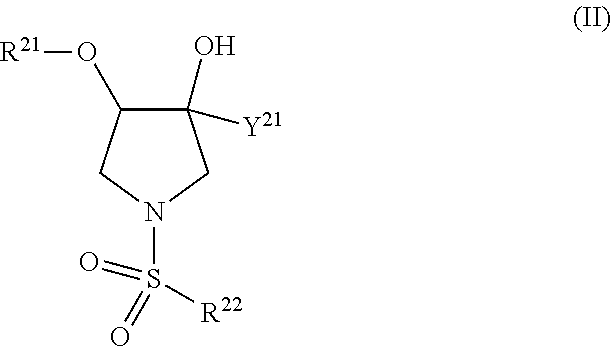
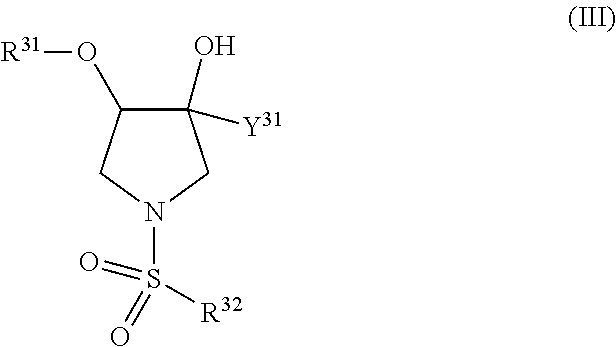
The present invention relates to pyrrolidine sulfonamide analogs (I), pharmaceutical compositions containing them and their use as TRPV4 antagonists.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Trpv4 antagonists
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
TRPV4 antagonists
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Methods of modulating angiogenesis via TRPV4
ActiveUS8394779B2Promote migrationSenses disorderNervous disorderIntracellular signallingCapillary Endothelium
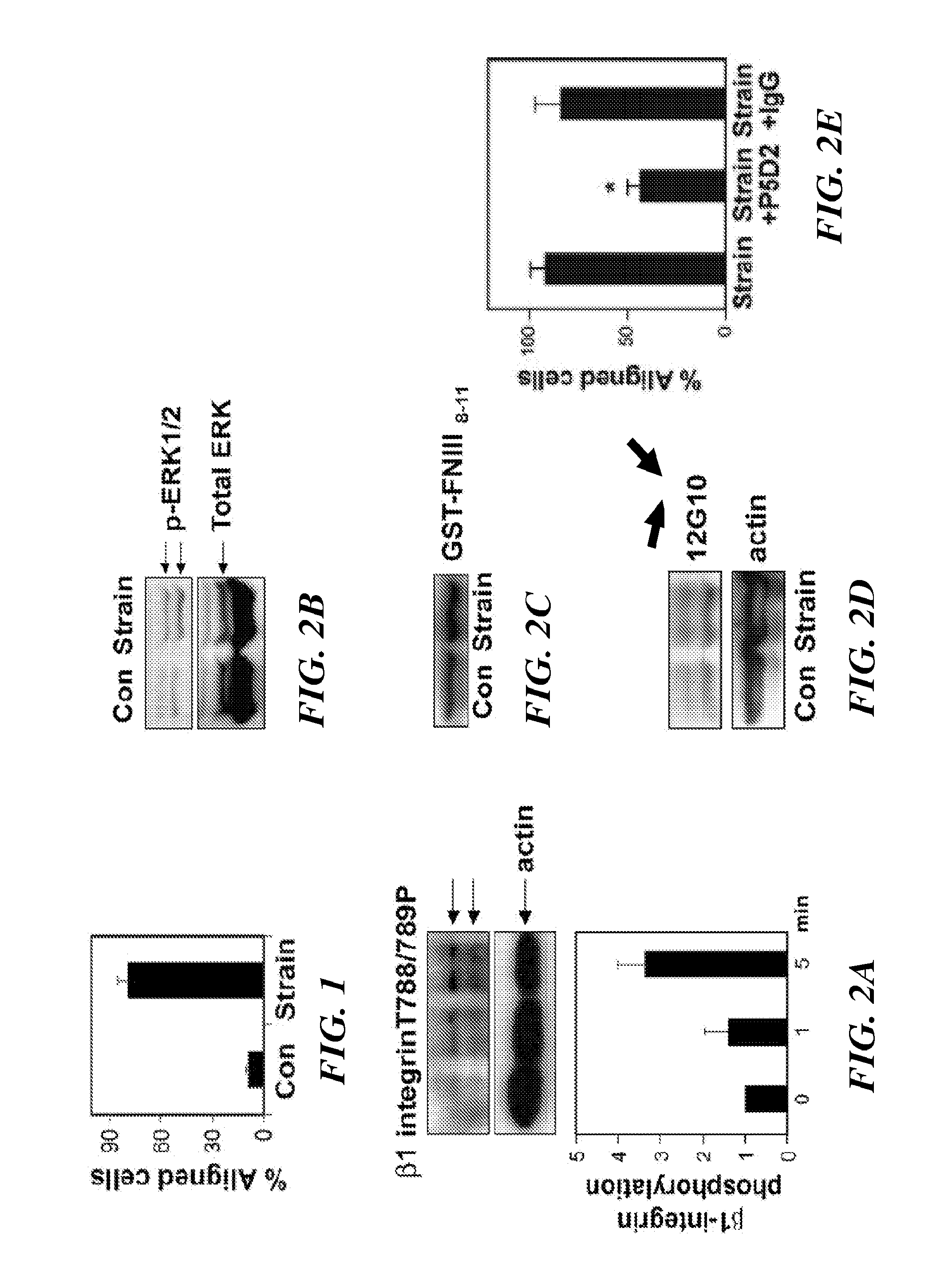
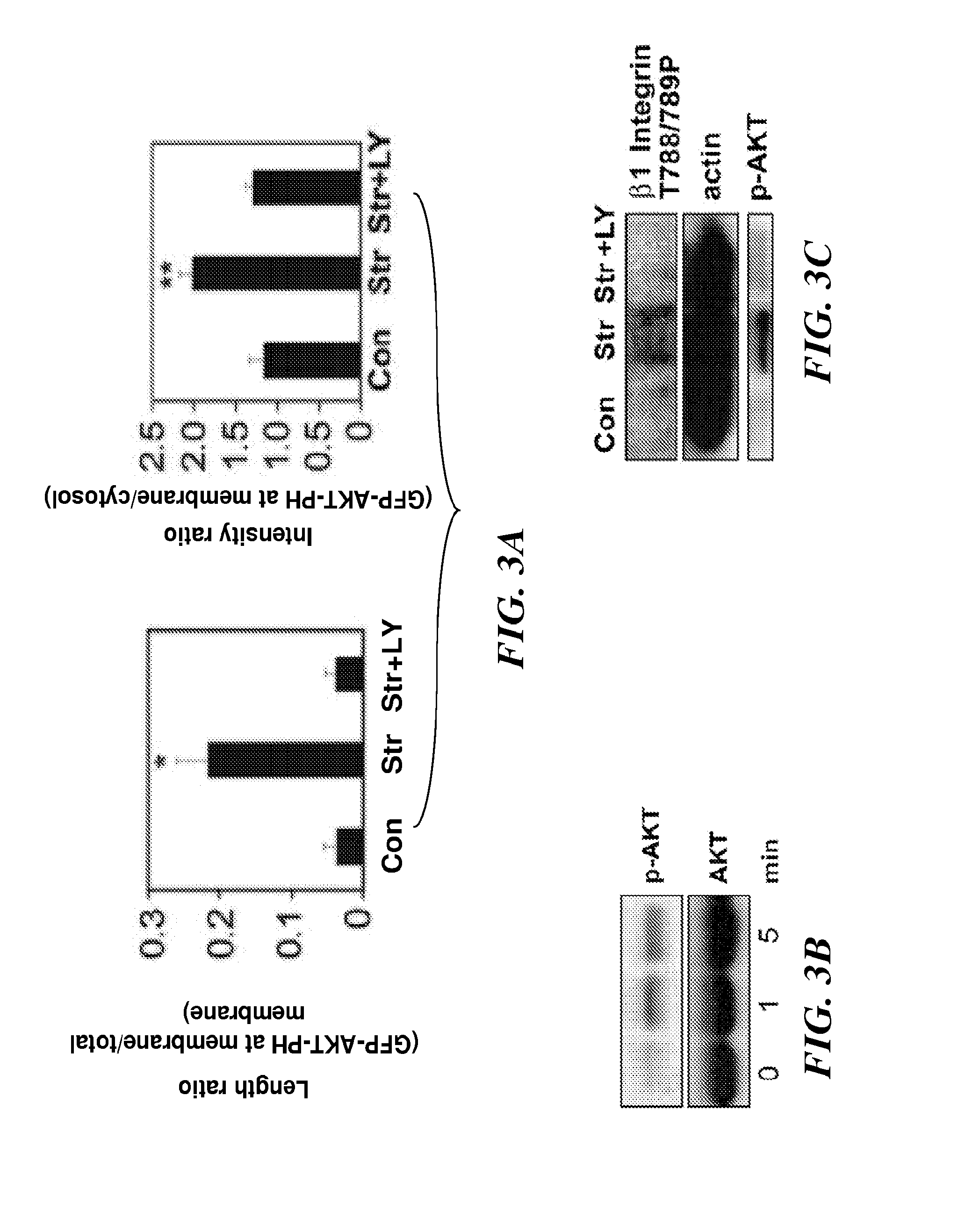
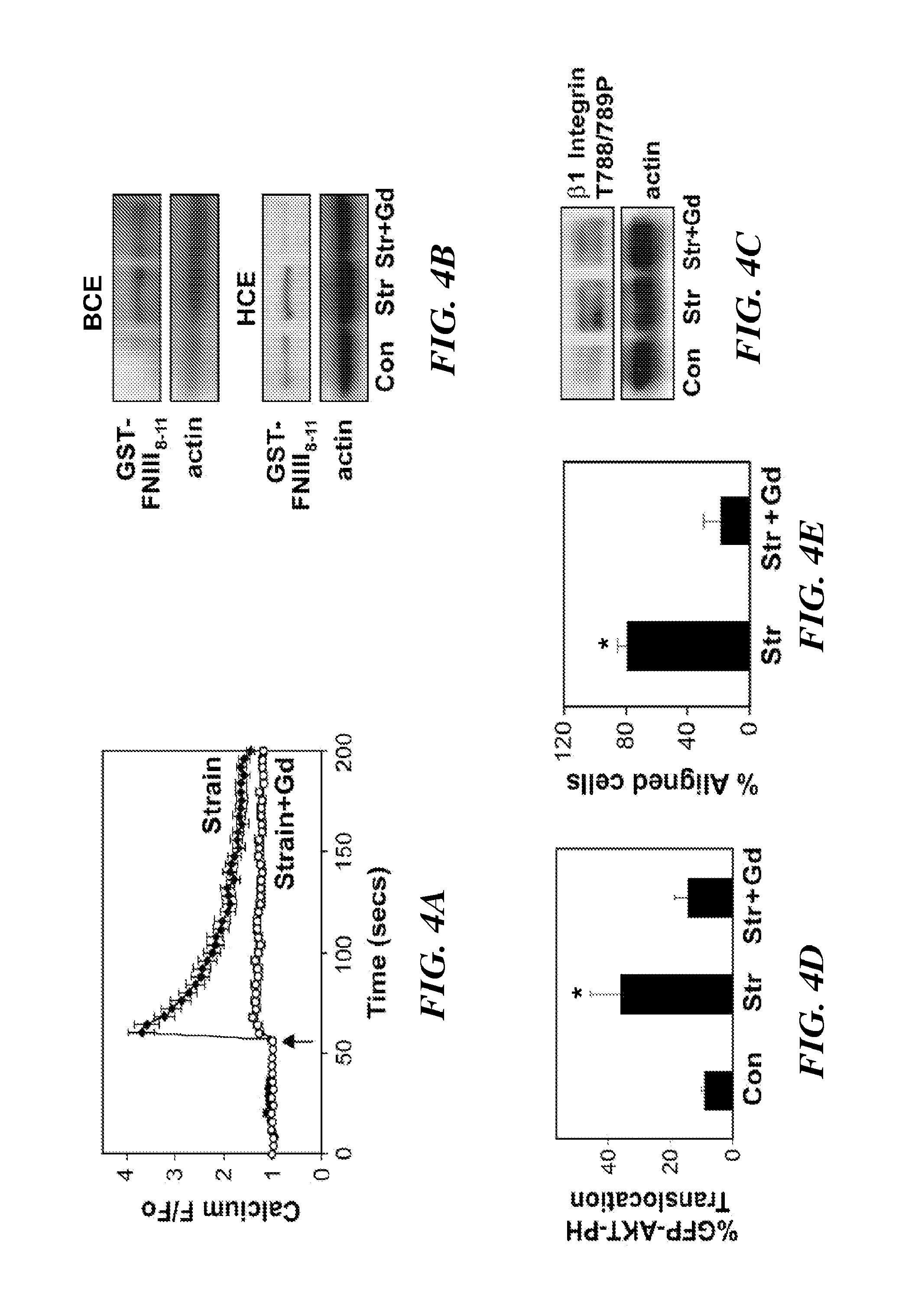
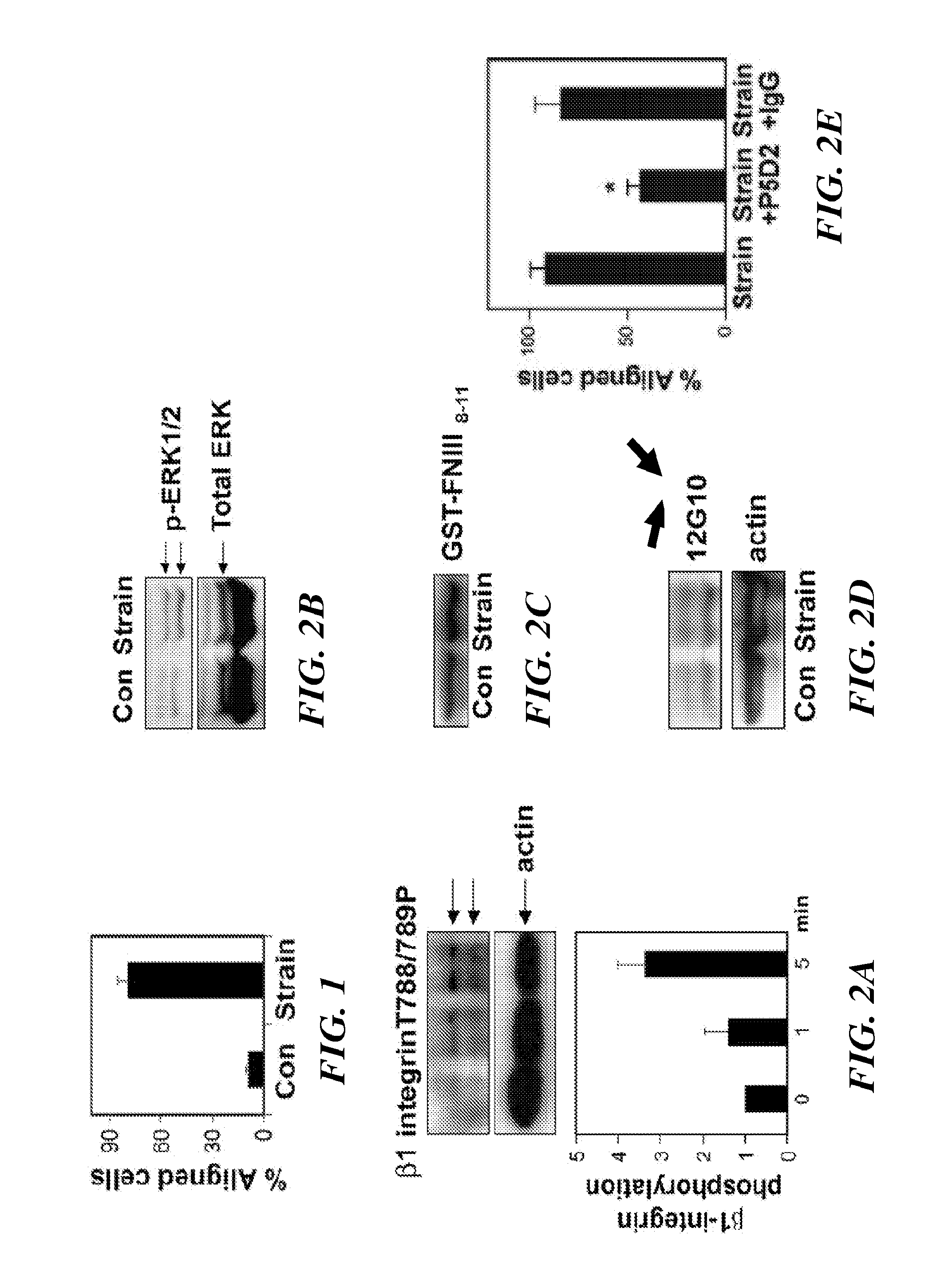
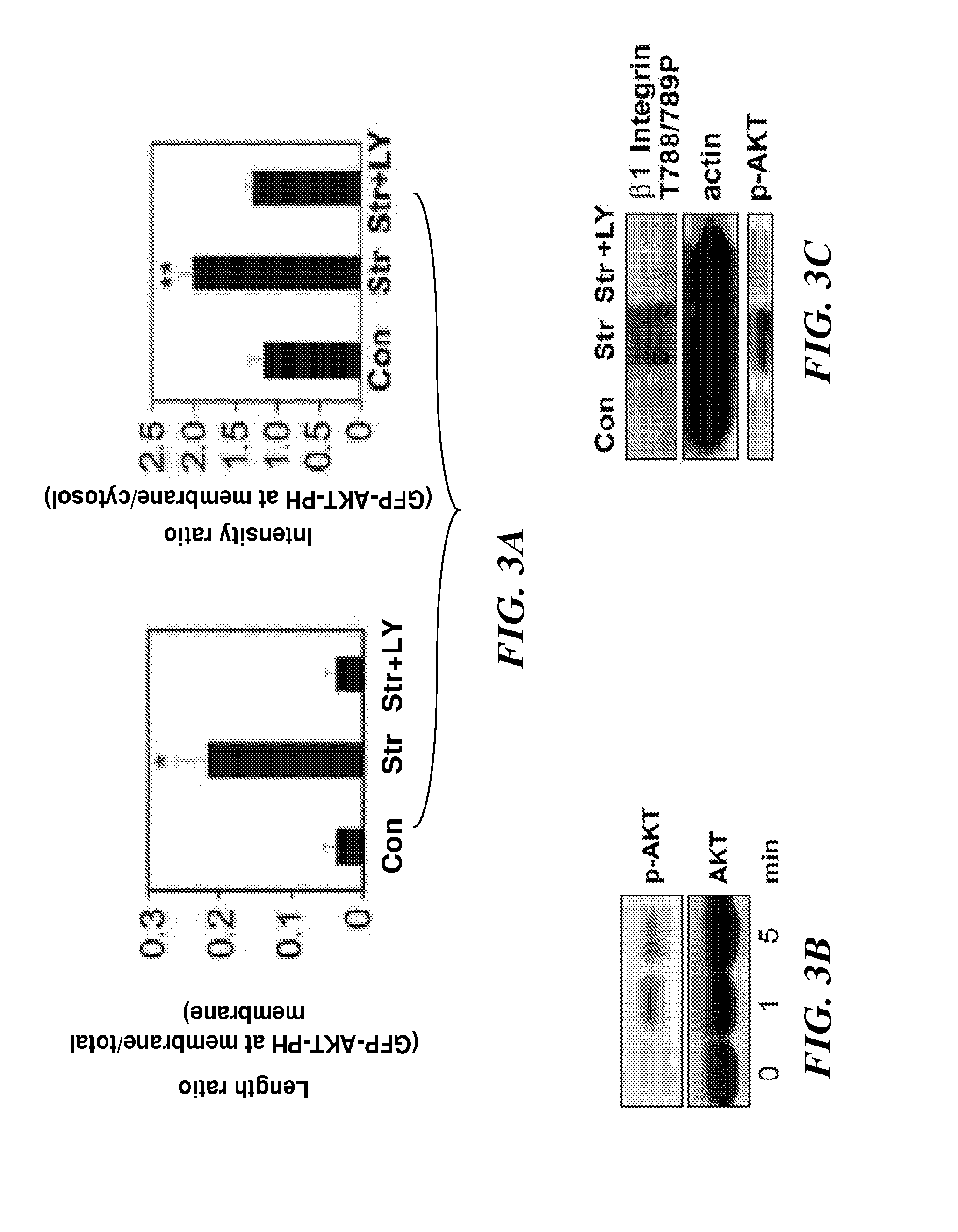
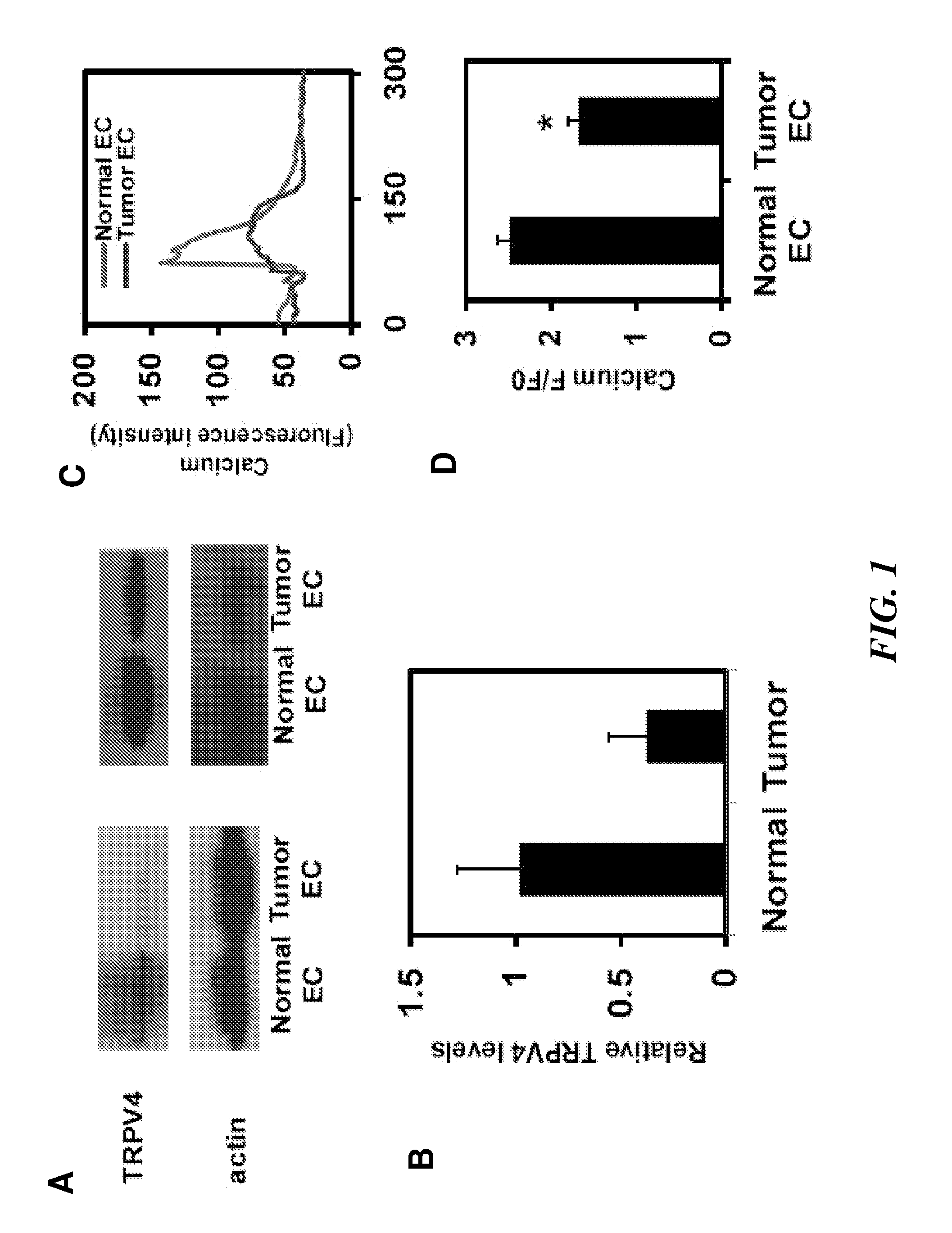
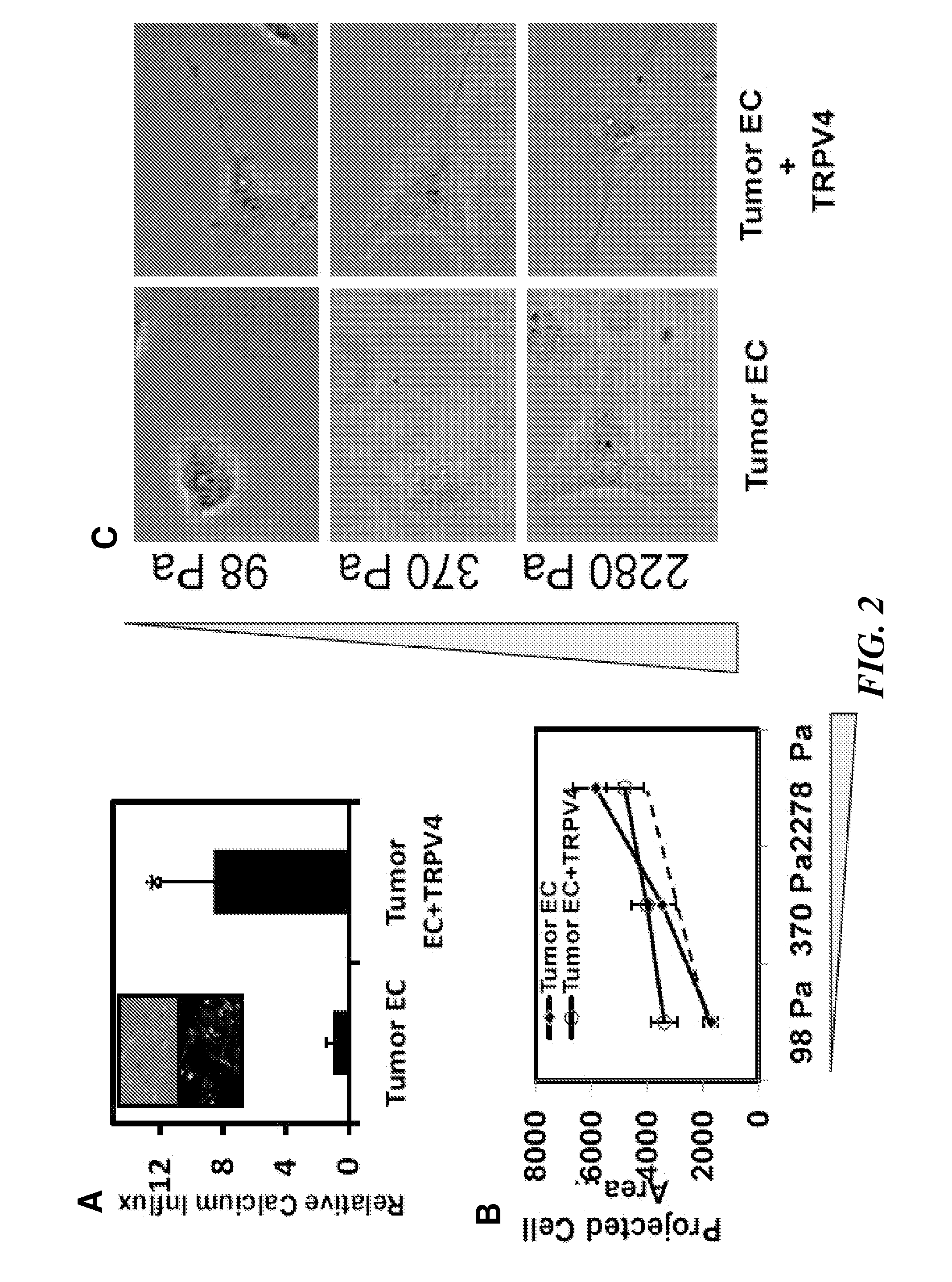
The present invention relates to methods of inhibiting capillary endothelial (CE) cell migration, the formation of CE networks and angiogenesis, and uses thereof for the purpose of treating angiogenesis-related diseases and disorders, particularly when the diseases or disorders are directly related aberrant angiogenesis. Inhibition is achieved by inhibiting TRPV4 activity, such as the levels of TRPV4 expression, calcium influx through TRPV4, and / or the intracellular signaling from TRPV4 via β1 integrin activation.
Owner:CHILDRENS MEDICAL CENT CORP
Protein involved in detection of cancer metastasis and a treatment thereof
InactiveUS20140199330A1High copy numberSsRNA viruses negative-senseCell receptors/surface-antigens/surface-determinantsCell PlasticityLymphatic Spread
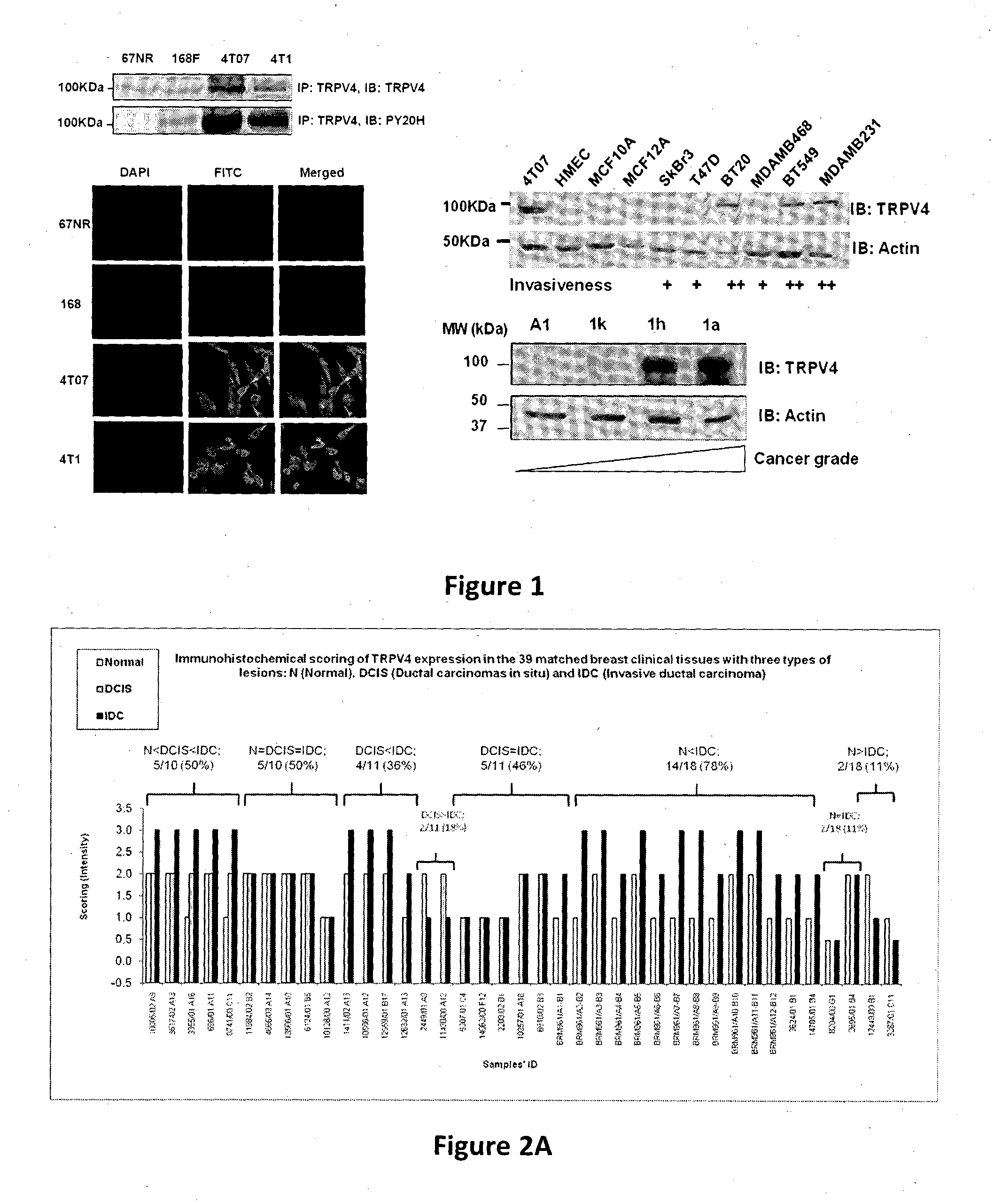
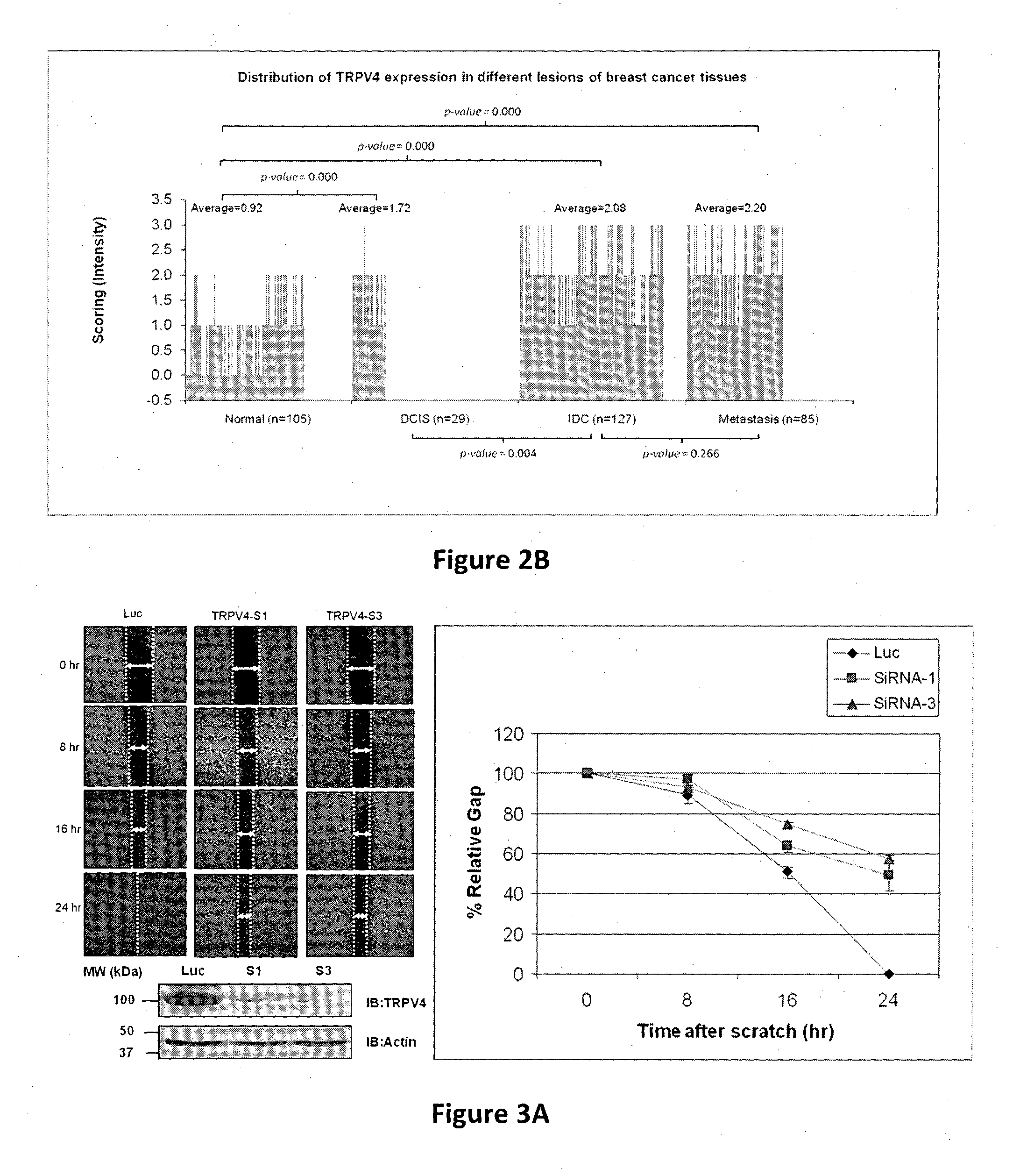
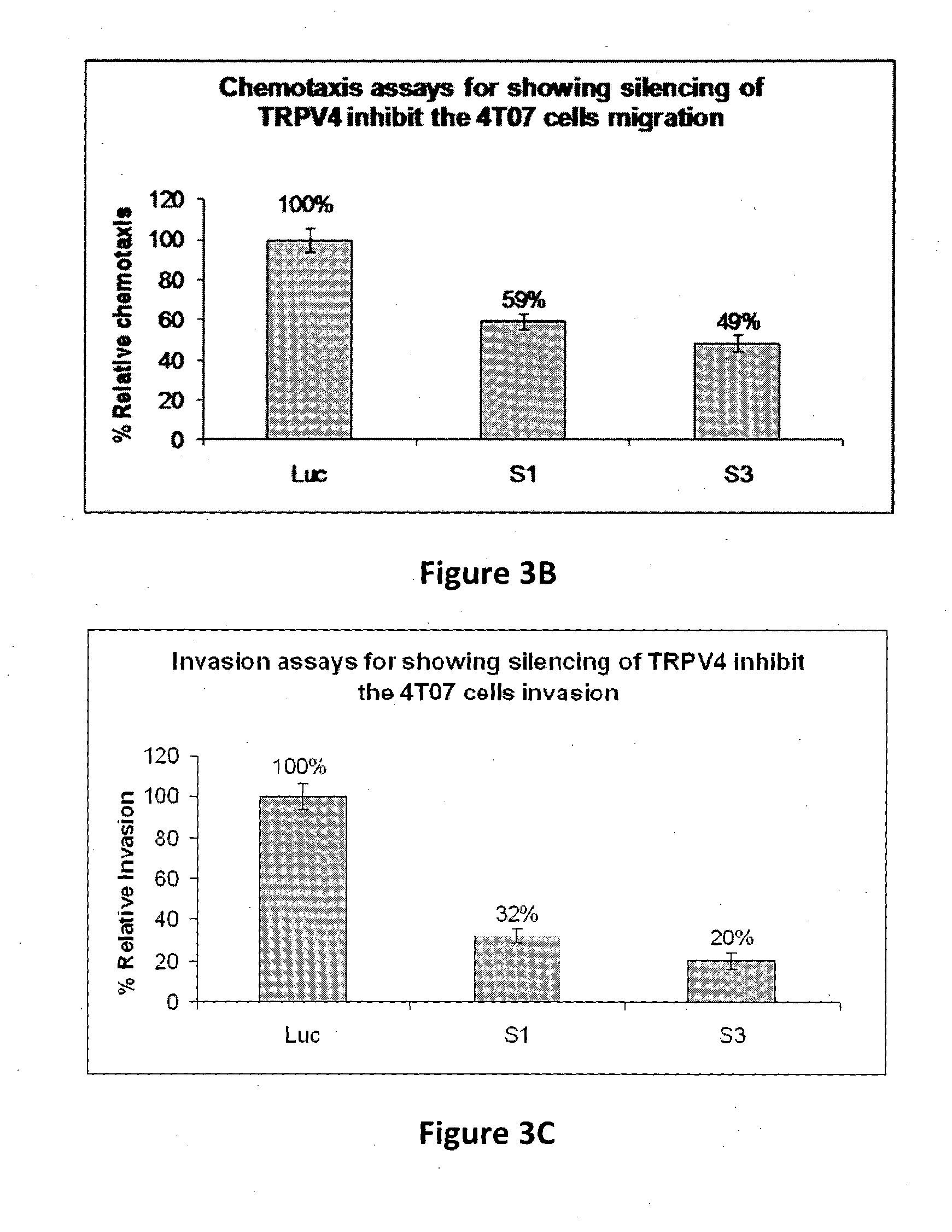
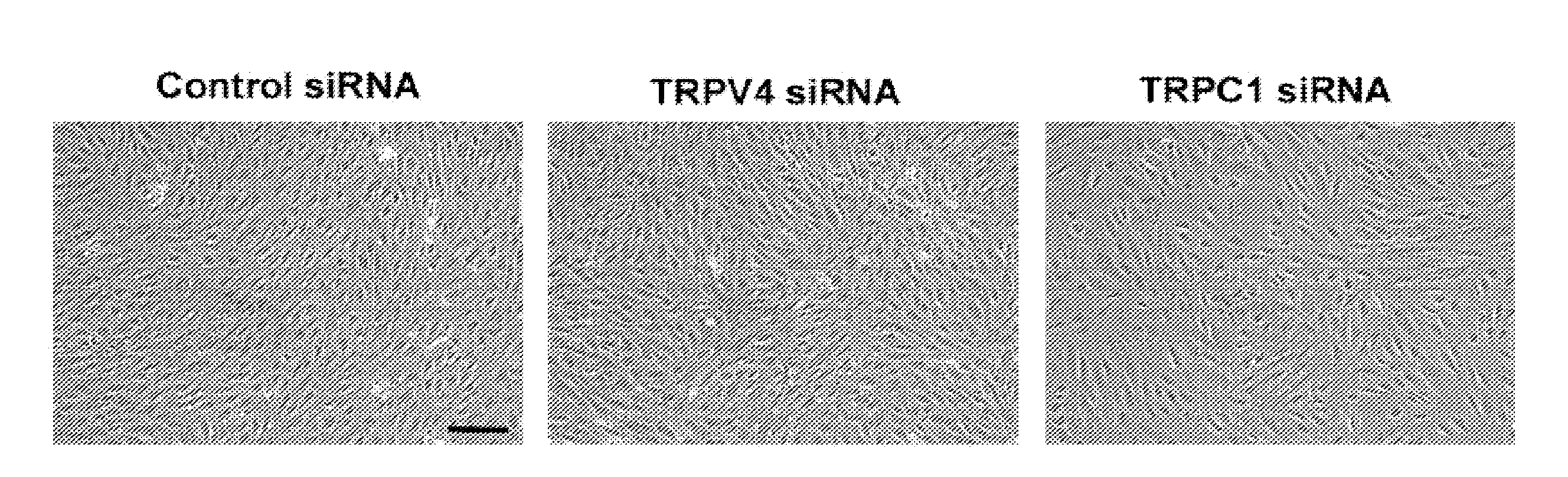
Using phosphoproteomics, we profiled the phosphorylation levels of hundreds of proteins concurrently across an isogenic model of breast cancer metastasis. Among them is TRPV4, a calcium channel that we found to be overexpressed in invasive breast tumors compared to ductal carcinoma in situ, a pre-neoplastic lesion and normal tissues. TRPV4 was also found to be elevated mostly in invasive breast cancer cell lines and less so in non-invasive breast cancer cell lines. These data led us to hypothesize that TRPV4 confer early traits of metastatic cancer cells. Functional studies revealed that silencing of TRPV4 expression diminished breast cancer cell migration and invasion significantly but not proliferation. Silencing expression of TRPV4 in metastatic breast cancer cells also reduced the number and size of metastatic colonies in mice. This supports the notion that TRPV4 is an attractive drug target to curb metastasis. Further experimentations suggested that the functional effect of TRPV4 on breast cancer cellular processes was associated with regulation of intracellular Ca2+, cell plasticity and expression of cell-cell adhesion proteins such as beta-catenin and E-cadherin. The latter two events have obvious implications in cancer invasion and intravasation / extravasation. We have also made novel observations that activation of TRPV4 by PDD led to activation of AKT and FAK pathways, both shown to be important to cell migration. In particular, downregulation of E-cadherin and b-catenin following TRPV4 activation has been shown to be mediated by the AKT pathway. Collectively, our data suggest that activation of Ca2+ dependent cascades and pathways associated with cell migration mediate TRPV4 function in breast cancer metastasis.
Owner:NAT UNIV OF SINGAPORE
Methods of modulating angiogenesis via trpv4
ActiveUS20110150894A1Reduce decreasePromote migrationSenses disorderNervous disorderIntracellular signallingDisease
The present invention relates to methods of inhibiting capillary endothelial (CE) cell migration, the formation of CE networks and angiogenesis, and uses thereof for the purpose of treating angiogenesis-related diseases and disorders, particularly when the diseases or disorders are directly related aberrant angiogenesis Inhibition is achieved by inhibiting TRPV4 activity, such as the levels of TRPV4 expression, calcium influx through TRPV4, and / or the intracellular signaling from TRPV4 via β1 integrin activation.
Owner:CHILDRENS MEDICAL CENT CORP
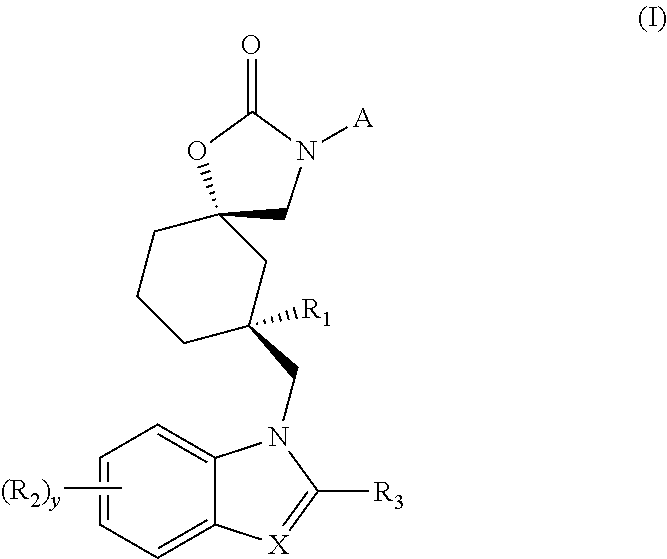
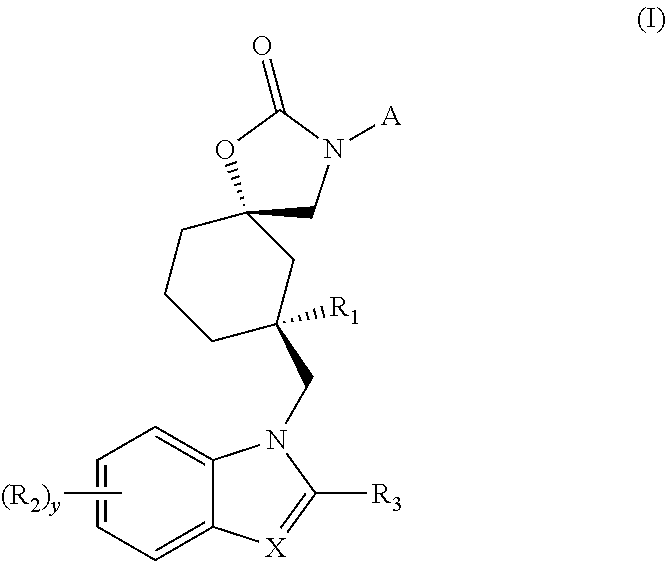
Trpv4 antagonist
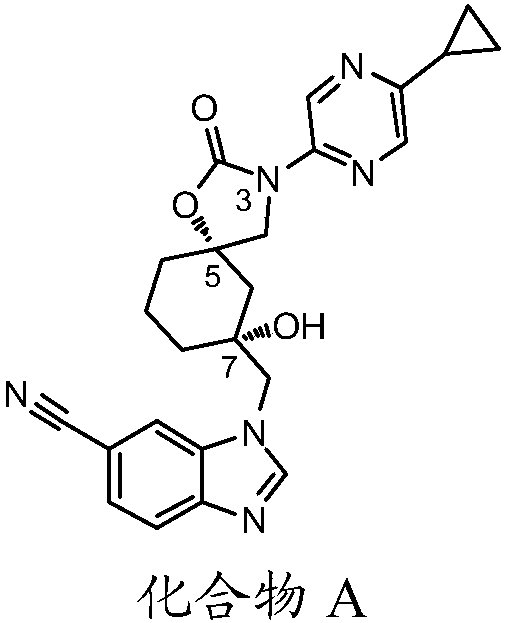
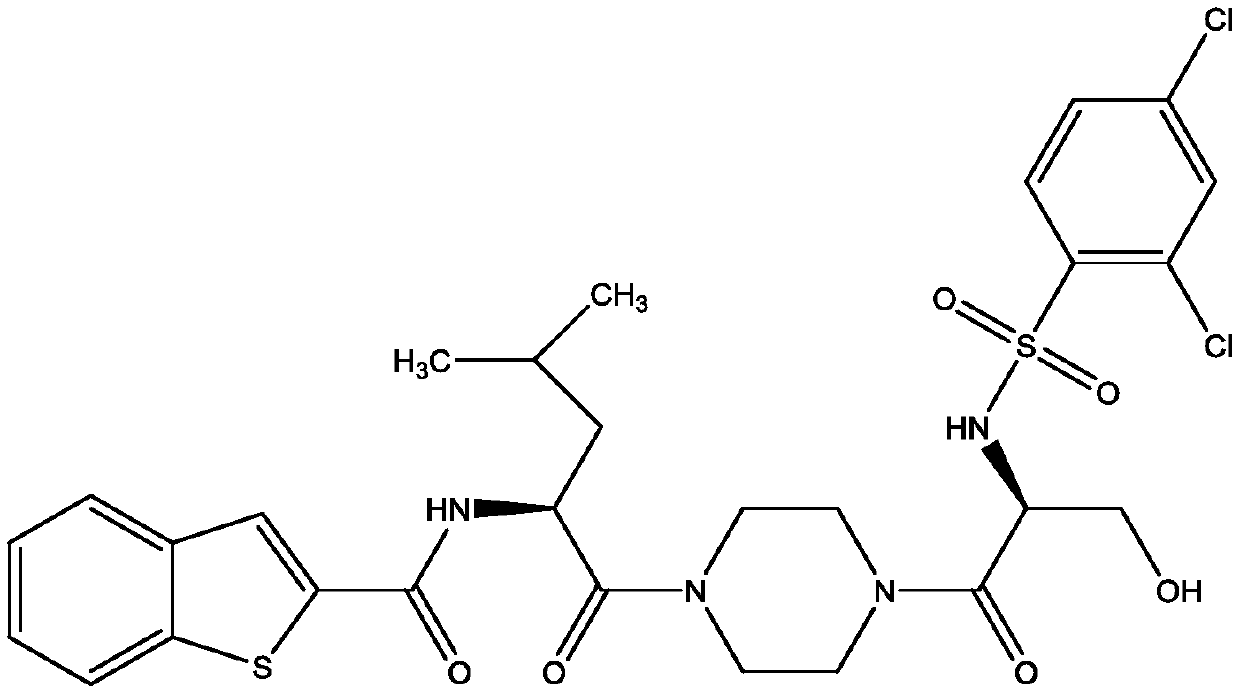
The present invention relates to a novel compound useful as a TRPV4 antagonist, specifically the compound 1-(((5S,7R)-3-(5-cyclopropylpyrazin-2-yl)-7-hydroxy-2-oxo-1-oxa-3-azaspiro[4.5]decan-7-yl)methyl)-1H-benzo[d]imidazole-6-carbonitrile, pharmaceutically acceptable salts thereof and pharmaceutical compositions containing the compound. The compound of the invention can be useful in the treatmentof a disease state selected from: atherosclerosis, disorders related to vasogenic edema, postsurgical abdominal edema, ocular edema, cerebral edema, local and systemic edema, fluid retention, sepsis,hypertension, inflammation, bone related dysfunctions and congestive heart failure, pulmonary disorders, chronic obstructive pulmonary disorder, ventilator induced lung injury, high altitude inducedpulmonary edema, acute respiratory distress syndrome, acute lung injury, pulmonary fibrosis, sinusitis / rhinitis, asthma, cough; including acute cough, sub-acute cough and chronic cough, pulmonary hypertension, overactive bladder, cystitis, pain, motor neuron disorders, genetic gain of function disorders, cardiovascular disease, renal dysfunction, stroke, glaucoma, retinopathy, endometriosis, pre-term labor, dermatitis, pruritus, pruritus in liver disease, diabetes, metabolic disorder, obesity, migraine, pancreatitis, tumor suppression, immunosuppression, osteoarthritis, crohn's disease, colitis, diarrhea, intestinal irregularity (hyperreactivity / hyporeactivity), fecal incontinence, irritable bowel syndrome (IBS), constipation, intestinal pain and cramping, celiac disease, lactose intolerance, and flatulence.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Trpa1 and trpv4 inhibitors and methods of using the same for organ-specific inflammation and itch
ActiveUS20170267651A1Compounds screening/testingOrganic active ingredientsDiluentDermatological disorders
Provided are methods of treating and / or preventing dermatological disorders. Provided are methods of reducing skin inflammation, reducing pain, and / or reducing itch in a subject in need thereof. The methods may include administering to the subject an effective amount of a TRPA1 and / or TRPV4 inhibitor. Further provided are compositions including a TRPA1 and / or TRPV4 inhibitor compound in combination with a carrier, vehicle, or diluent that is suitable for topical application.
Owner:RGT UNIV OF CALIFORNIA +1
Novel applications of TRPV4 agonist GSK1016790A
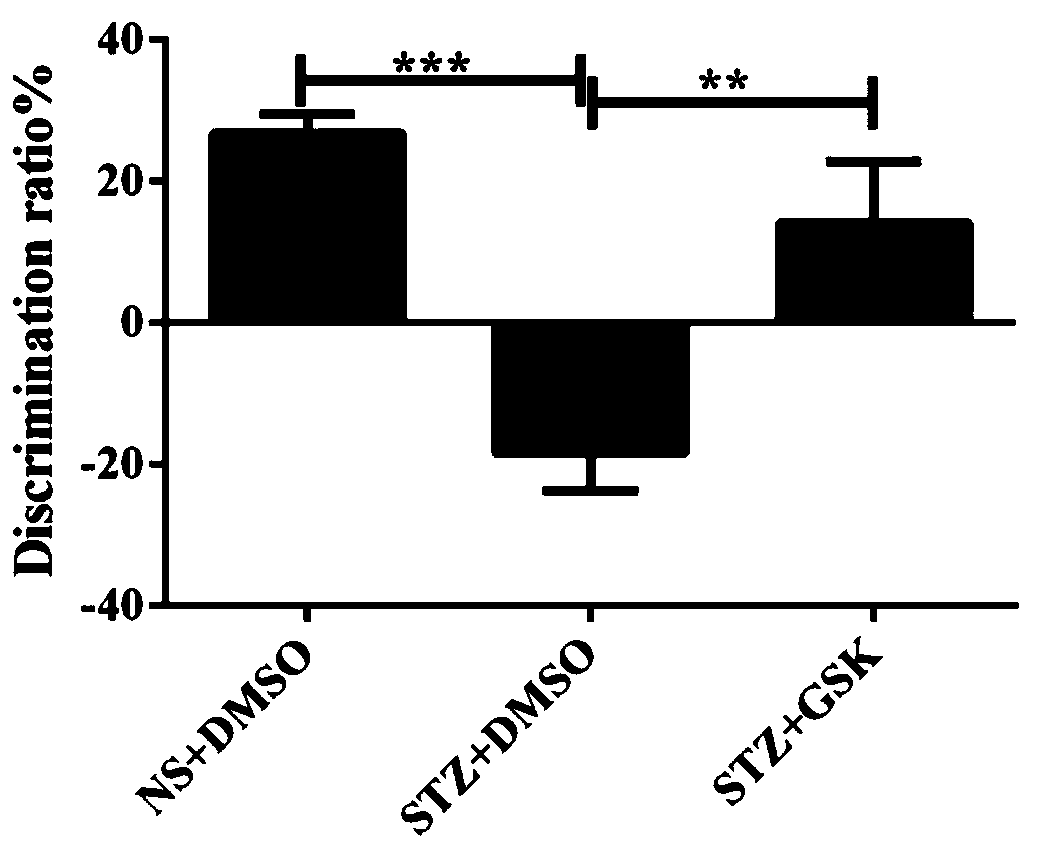
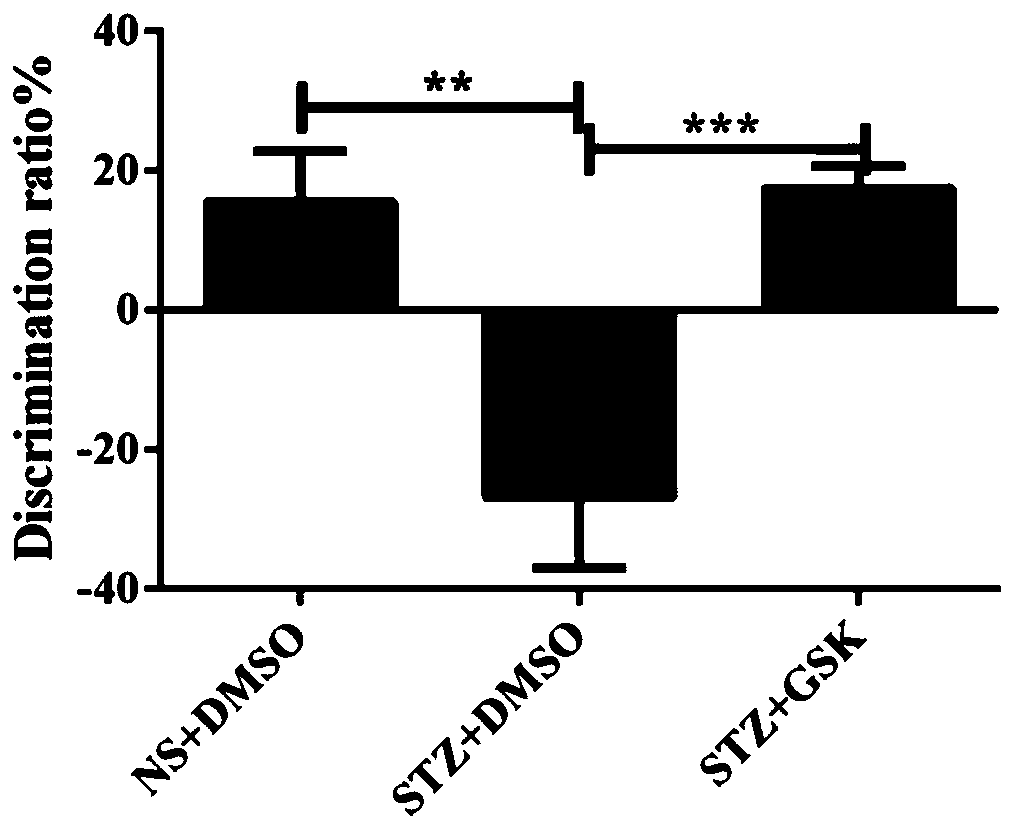
InactiveCN111195253AImprove preferenceIncreased ability to recognize new objects in mice with impaired learning and memoryOrganic active ingredientsNervous disorderDiabetes mellitusPharmaceutical drug
The invention belongs to the field of medicines, discloses new applications of a TRPV4 agonist GSK1016790A, and particularly relates to an application of the TRPV4 agonist GSK1016790A in the preparation of a medicine for treating diabetes cognitive decline. Experiments show that the TRPV4 agonist GSK1016790A can obviously improve the recognition capability of STZ-induced learning and memory impairment mice on new objects, can obviously increase the preference of mice to new positions, can be used for preparing the medicine for treating diabetes cognitive decline, and has high clinical application value and development prospect.
Owner:NANHUA UNIV
Compound for specifically enhancing spatial coupling degree of TRPV4-KCa2.3 complex and application of compound
ActiveCN112390782AHigh activityAvoid poor resultsOrganic active ingredientsOrganic chemistryChemical compoundVasodilation
The invention discloses a compound for specifically enhancing the spatial coupling degree of a TRPV4-KCa2.3 complex and an application of the compound, and belongs to the field of chemical medicines.The compound with the structure represented by the general formula (I) is mainly used for uncoupling TRPV4 and KCa2.3 in hypertensive patients, so that two uncoupled proteins can be recoupled, and thenormal state of the two uncoupled proteins can be recovered. Under the normal condition, according to TRPV4 and KCa2.3, TRPV4 receive signals of calcium ions, due to calcium ion inflow, potassium ionoutflow of a downstream KCa2.3 channel is stimulated, vasodilation is achieved through depolarization, and thus the blood pressure of the hypertensive patients is recovered to the normal level.
Owner:JIANGNAN UNIV
Method for preparing medicine for reducing coupling degree of TRPV4 and NOX2
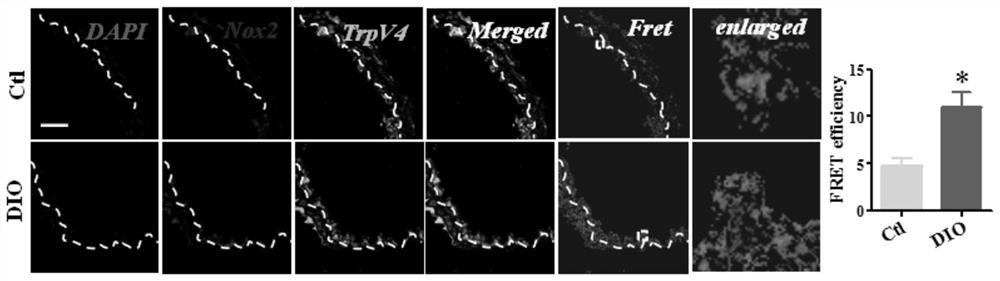
PendingCN112336723AStrong targetingRealize local adjustmentOrganic active ingredientsNervous disorderAntioxidative stressGambogic acid
The invention discloses a method for preparing a medicine for reducing a coupling degree of TRPV4 and NOX2, and belongs to the technical field of chemical medicines. According to the invention, entrectinib, L755507, gambogic acid and M12 compounds are used as active ingredients and applied to preparing the medicine for reducing the coupling degree of TRPV4 and NOX2, anti-oxidative stress and vascular permeability, in order to realize a reduced coupling degree in obese patients with excessively coupled TRPV4 and NOX2, so that the two excessively coupled proteins are decoupled, and the normal state is recovered, thus oxidative stress and vascular permeability of the obese patients are recovered to normal levels.
Owner:JIANGNAN UNIV
Application of medicine in preparation of drugs for treating metastatic encephaloma and related diseases thereof
ActiveCN111773390AHas antitumor effectSmall side effectsHydroxy compound active ingredientsDigestive systemDiseaseSequela
This application provides an application of a medicine in the preparation of drugs for treating metastatic encephaloma and related diseases thereof. The medicine includes at least one TRPV4 agonist capable of promoting calcium ion influx of a TRPV4 signal channel, and the metastatic encephaloma and related diseases thereof include metastatic encephaloma, diseases arising during the formation of metastatic encephaloma, complications and sequelae caused by metastatic encephaloma, and diseases related to metastatic encephaloma. The medicine is applied in the preparation of the drugs for treatingmetastatic encephaloma-related diseases, a significant anti-tumor effect can be played through a clear mechanism of action, the therapeutic effects are good, and the application prospects are good.
Owner:NANJING UNIV
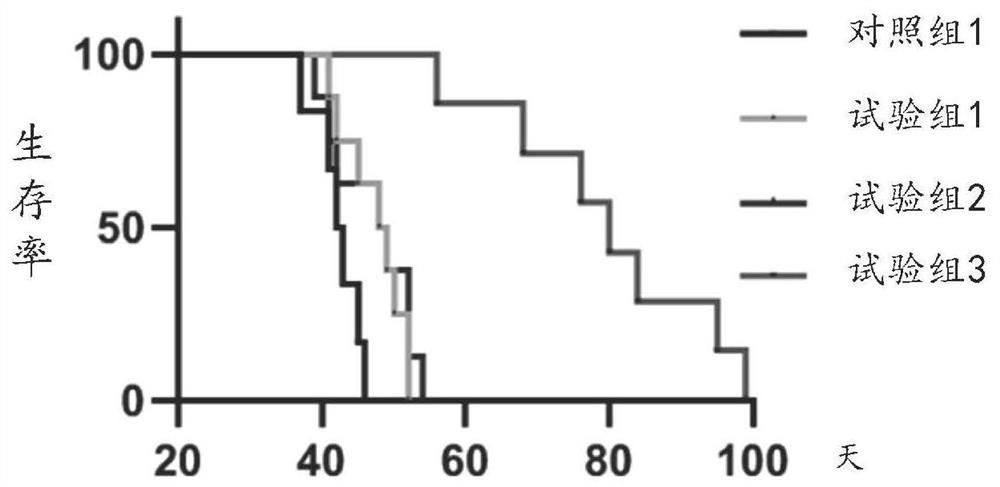
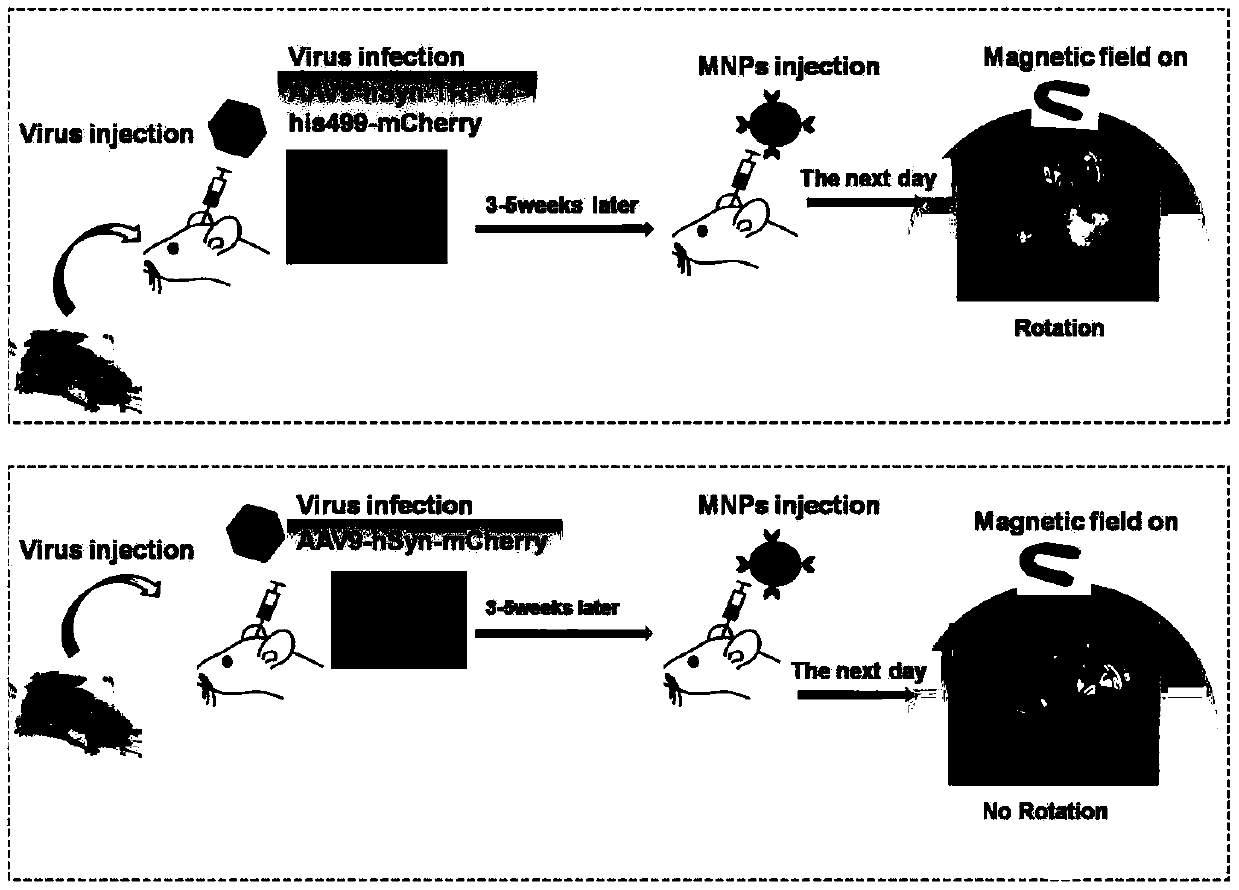
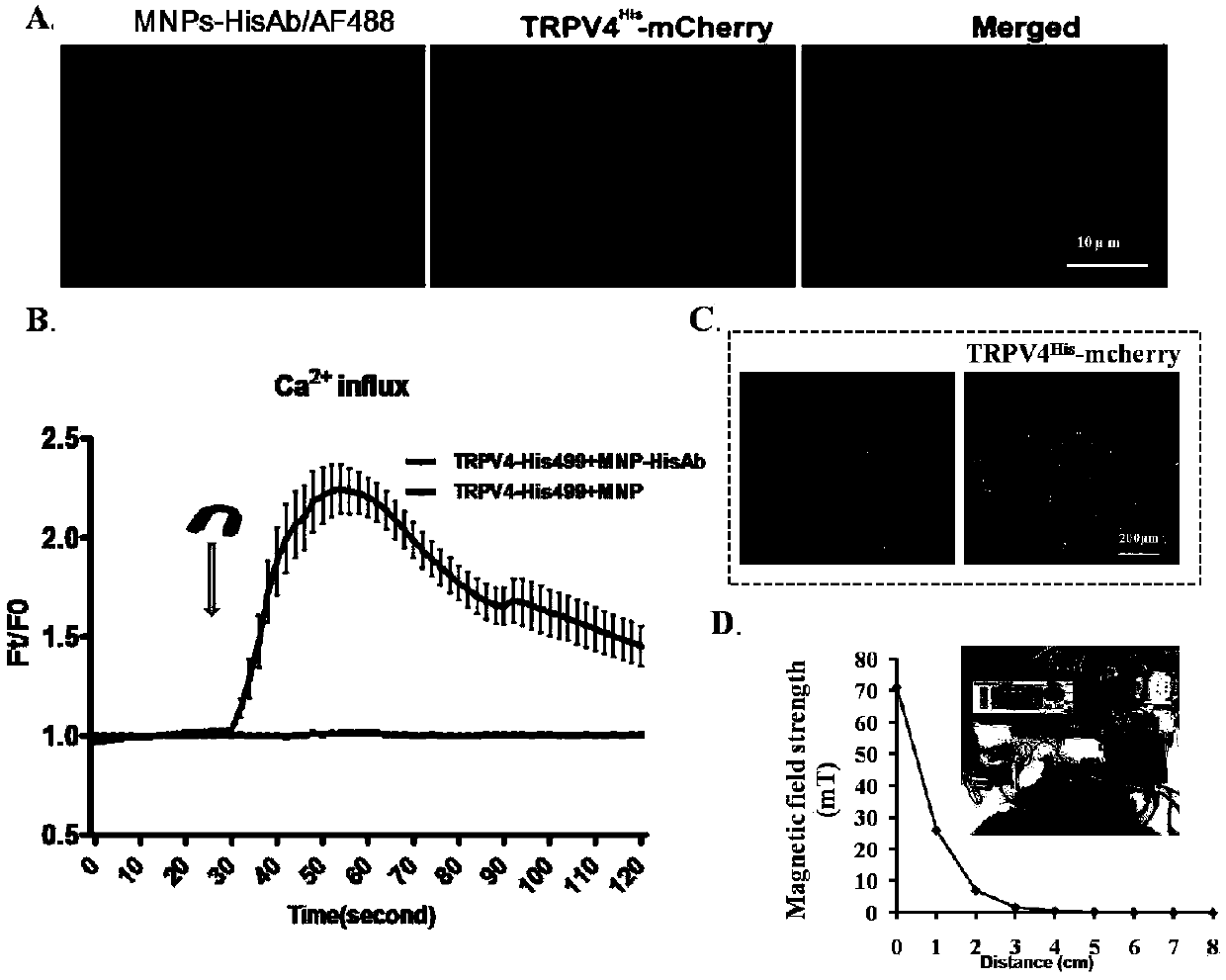
TRPV4-His499 protein sensitive to magnetic force, magnetic regulation tool and application
ActiveCN109651499AInduced calcium influxRegulate specific behaviorHybrid peptidesAnimals/human peptidesMagnetic tension forceMagnetite Nanoparticles
The invention provides a TRPV4-His499 protein sensitive to magnetic force, a magnetic regulation tool and application, and relates to the technical field of system nerve science. TRPV4-His499 proteinis capable of sensing magnetic fields and being activated with the aid of functionally modified magnetic nanoparticles, followed by triggering cellular calcium influx for neurocalcium signaling. The specific operation is as follows: inserting a 6*his label at the 499th position amino acid position of the TRPV4 protein to obtain the TRPV4-His499 protein sensitive to magnetic force, transferring theTRPV4-His499 protein into the brain of a mouse by using virus, simultaneously injecting magnetic nanoparticles wrapped by anti-His antibodies, and inducing cell calcium influx under the action of a magnetic field, can be used for conducting nerve calcium signal and regulating mouse behaviors.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Drug for inhibiting brain tumor and application thereof
ActiveCN111920788AGood inhibitory effectImprove anti-tumor activityNervous disorderHydroxy compound active ingredientsDiseaseSide effect
The invention provides a drug for inhibiting brain tumor and application thereof. The drug for inhibiting the brain tumor comprises at least one TRPV4 agonist capable of promoting calcium ion internalflow of a TRPV4 signal channel. The drug has a good treatment effect on brain tumors and related diseases thereof, and has a good application prospect in treatment of brain tumors and related diseases thereof. The drug is applied to medicines for treating brain tumors and related diseases thereof, can reduce toxic and side effects generated in the clinical treatment process of the brain tumors and the related diseases thereof and improve the treatment effect of the brain tumors and the related diseases thereof.
Owner:LVCHAN BIOTECHNOLOGY CO LTD
Efficacy of cancer therapy
ActiveUS20130150432A1More cell deathReduced calcium influxBiocidePeptide/protein ingredientsCell signaling pathwaysCancer therapy
Embodiments of the invention provide a method of improving the efficacy of an anti-cancer therapy and a method of treatment of cancer by normalizing angiogenesis in cancer. By enhancing the cell signaling pathway via a TRPV4 receptor in tumor endothelial cells, either by a TRPV4 agonist or by increasing the expression of TRPV4 in the tumor endothelial cells, the tumor endothelial cells behave normally and form normal angiogenic network for better anti-cancer therapy to the tumors.
Owner:NORTHEAST OHIO MEDICAL UNIV +1
TRPV4 antagonists
The present invention relates to pyrrolidine sulfonamide analogs, pharmaceutical compositions containing them and their use as TRPV4 antagonists.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Recombinant TRPV4 protein and preparation method thereof
The invention provides a recombinant TRPV4 protein and a preparation method thereof. The recombinant TRPV4 protein contains a TRPV4 protein, a GST tag and a 6his tag. Compared with the prior art, the method has the beneficial effects that the GST tag and the 6his tag are added to the TRPV4 protein at the same time, so that complete TRPV4 protein fragments can be kept in the preparation process of the recombinant TRPV4 protein.
Owner:HUBEI UNIVERSITY OF MEDICINE
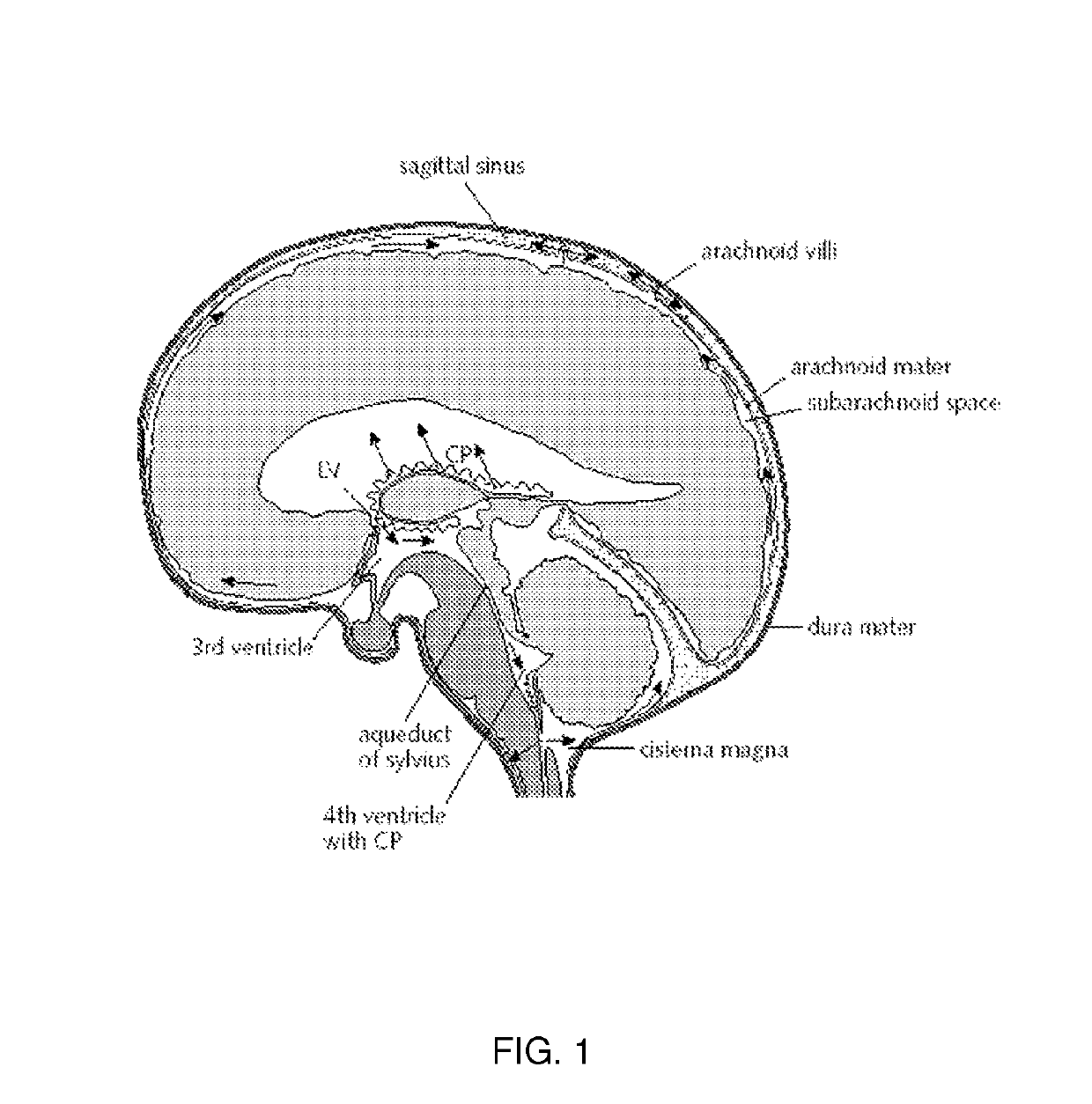
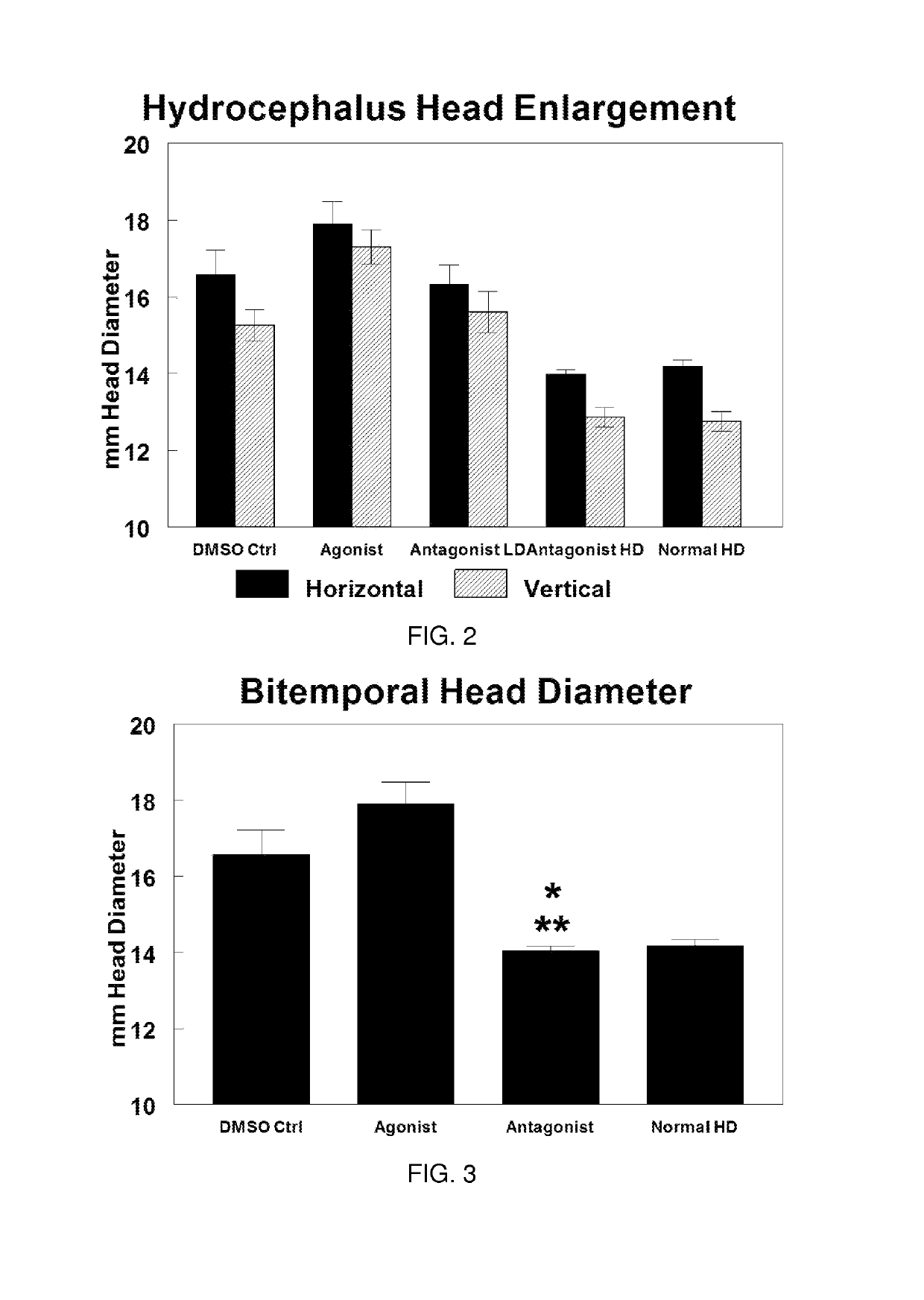
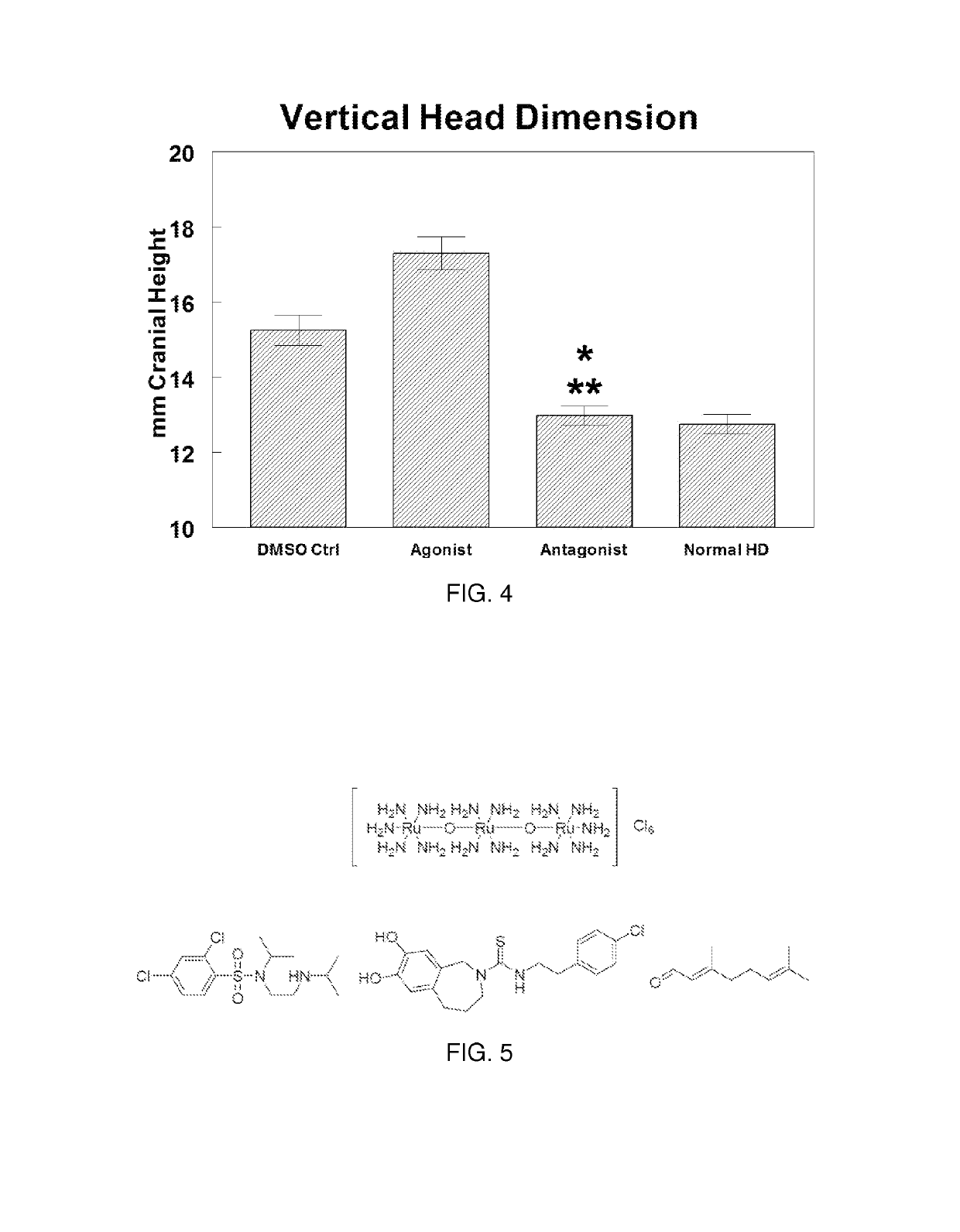
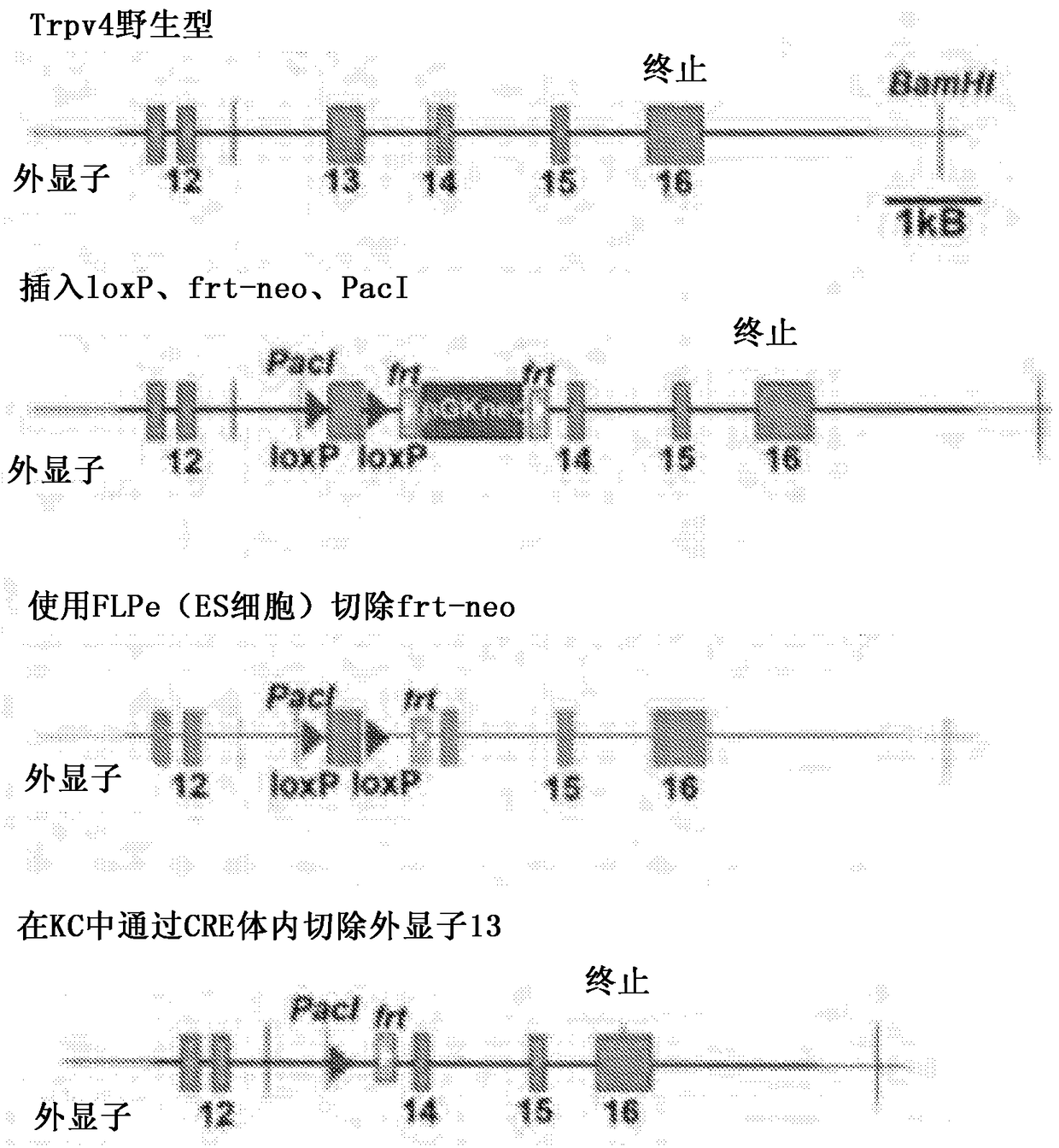
Use of TRPV4 antagonists to ameliorate hydrocephalus and related materials and methods
Owner:INDIANA UNIV RES & TECH CORP
Novel compounds
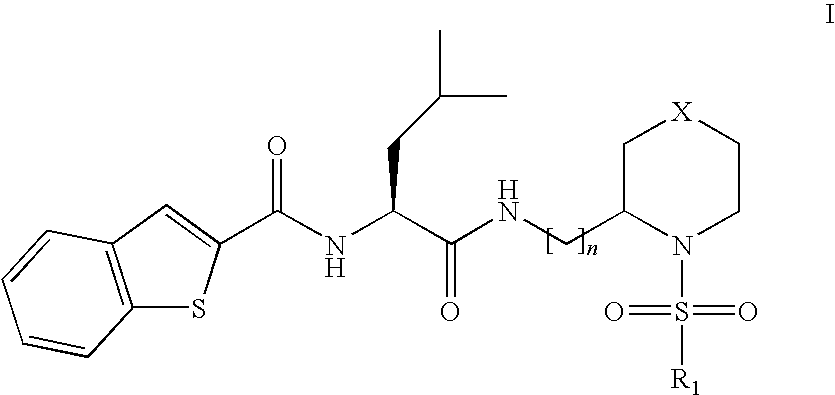
This invention relates to novel compounds useful in the treatment of diseases associated with TRPV4 channel receptor. More specifically, this invention relates to certain substituted piperidines, according to Formula ISpecifically, the invention is directed to compounds according to Formula IwhereinR1 is optionally substituted aryl;X is CH2, S, or SO2; andn=1 or 2.
Owner:SMITHKLINE BECKMAN CORP
A compound that specifically enhances the spatial coupling degree of trpv4-kca2.3 complex and its use
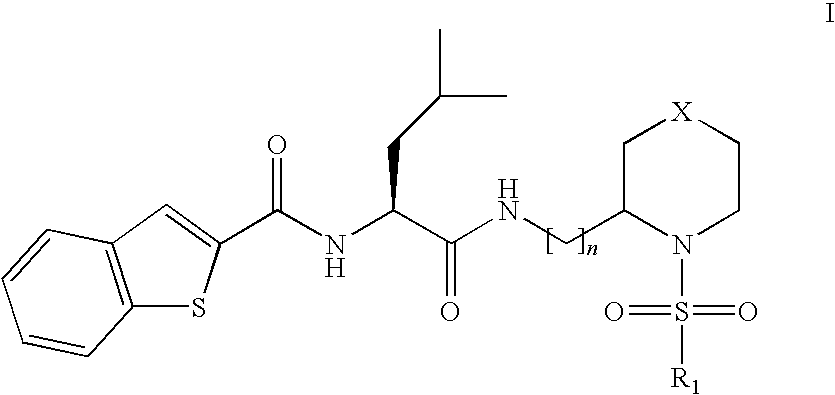
ActiveCN112390782BHigh activityAvoid poor resultsOrganic active ingredientsOrganic chemistryPotassium ionsBiochemistry
The invention discloses a compound that specifically enhances the spatial coupling degree of a TRPV4-KCa2.3 complex and an application thereof, belonging to the field of chemical medicine. The compound with the structure of general formula (I) of the present invention is mainly aimed at the uncoupling of TRPV4 and KCa2.3 in hypertensive patients, which can recouple the two uncoupled proteins to restore the normal state. Under normal circumstances, TRPV4 and KCa2.3 receive calcium ion signals from TRPV4. Due to the influx of calcium ions, it stimulates the outflow of potassium ions from the downstream KCa2.3 channel. return to normal levels.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com