Method for preparing medicine for reducing coupling degree of TRPV4 and NOX2
A drug and coupling technology, applied in the field of chemical medicine, can solve the problems of strong side effects and off-target, and achieve the effect of small side effects, strong targeting, and prevention of thrombosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: In vitro coupling activity test of compounds
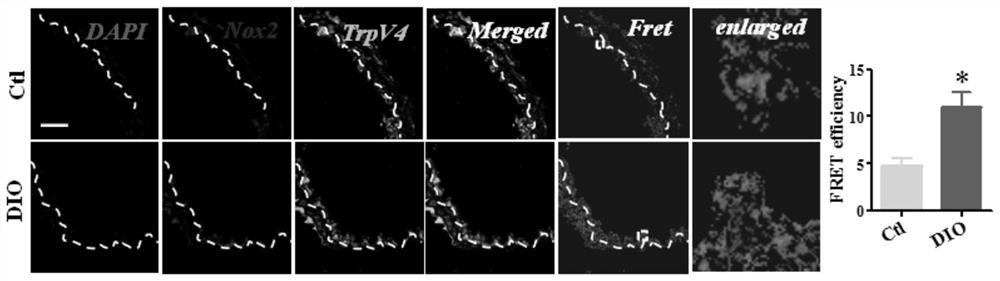
[0068] Experimental method: HEK-293 cells co-transfected with TRPV4 and NOX2 or co-transfected with AQP1 and NOX2 were treated and planted in the center of the confocal small dish. When the confluence of the cells reached about 80%, DMSO (1‰) was used respectively. Tretinib (10nM), L755507 (10μM), Gambogic acid (1μM), CB-839 (1μM), Bohemine (1μM), M12 (10nM) were incubated for 24 hours, fixed with 4% paraformaldehyde for 20 minutes, Permeabilize with PBS containing 0.1% Triton X-100 for 10 minutes, and block with PBS containing 5% BSA for half an hour.
[0069] Cells were then stained with TRPV4-550 and NOX2 as primary and conjugated secondary antibodies (invitrogen) and with DAPI (Vector Laboratories, Burlingame, CA, USA). Shoot with a laser confocal microscope, and calculate the Fret value. For specific results, see Figure 5 and Table 1.
[0070] Table 1 Effects of different compounds on the coupling of TR...
Embodiment 2
[0076] Example 2: Exploring the Effect of Compounds on Intracellular ROS Levels
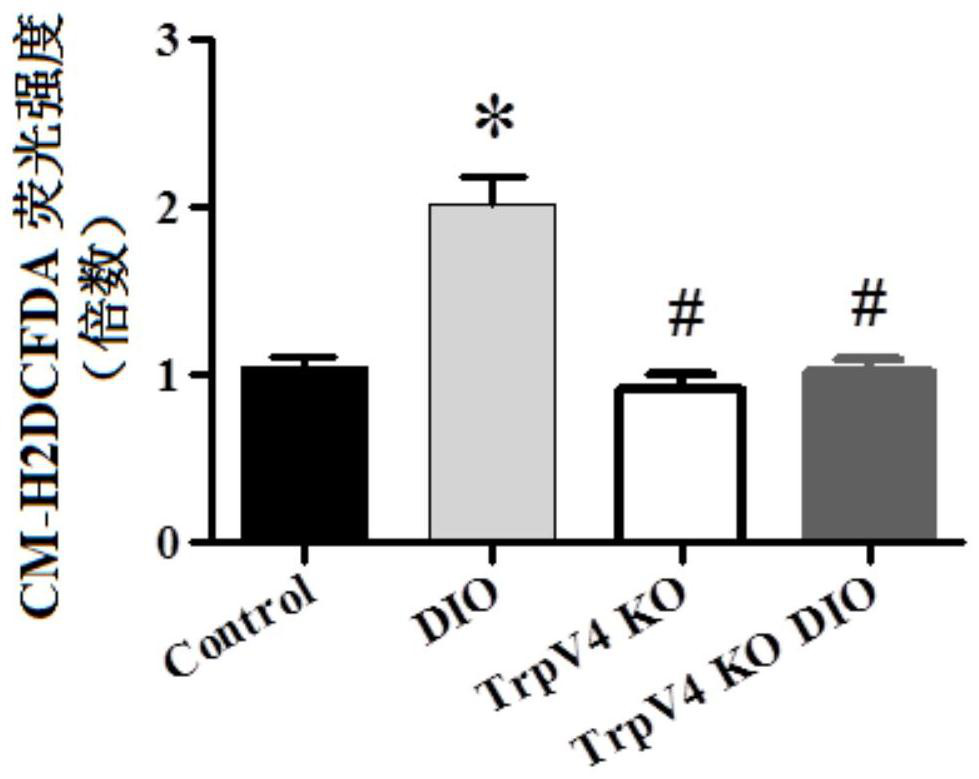
[0077] Experimental method: The isolated obese mouse primary endothelial cells were treated and planted in the center of the confocal small dish. When the confluence of the cells reached about 80%, they were incubated with 10Nm M12 for 24 hours, and the medium was discarded and washed with NPSS. After three trips, cells were incubated with NPSS containing CM-H2DCFDA in a dark environment in an incubator at 37 °C. Then use a laser confocal microscope to shoot, and use image J to calculate the fluorescence intensity statistically, which can reflect the level of intracellular ROS. For specific results, see Image 6 and Table 2.
[0078] The influence of table 2 compound on intracellular ROS level
[0079] CM-H2DCFDA fluorescence intensity (multiple compared with DIO) Unincubated DIO group 1.000000 Incubate with M12 0.496864
[0080] Where: DIO means diet-induced obesi...
Embodiment 3
[0082] Example 3: Exploring the effects of compounds on the cellular structure of obese mice:
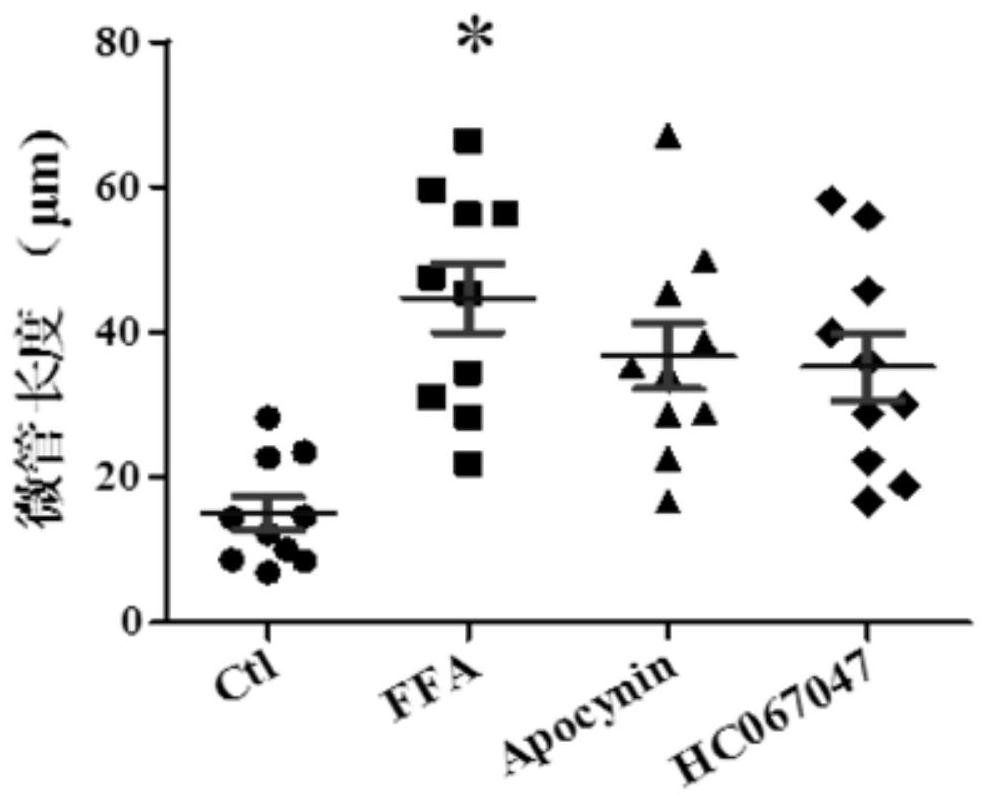
[0083] Experimental method: Isolate the primary endothelial cells of obese mice, plant them in the center of the confocal small dish after treatment, and when the confluence of the cells reaches about 80%, incubate with 10nM M12 for 24 hours, discard the medium, and wash three times with NPSS Finally, fix with 4% paraformaldehyde for 20 minutes, permeabilize with PBS containing 0.1% Triton X-100 for 10 minutes, and block with PBS containing 5% BSA for half an hour. Cells were incubated with NPSS mixed with rhodamine-Phallotoxins (invitrogen) for half an hour and stained with DAPI (Vector Laboratories, Burlingame, CA, USA). Images were taken with a confocal laser microscope. For specific results, see Figure 7
[0084] Where: BSA refers to bovine serum albumin. PBS is a buffer solution. Rhodamine-Phalloidin is a high-affinity probe of F-actin, which can be used to observe the ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com