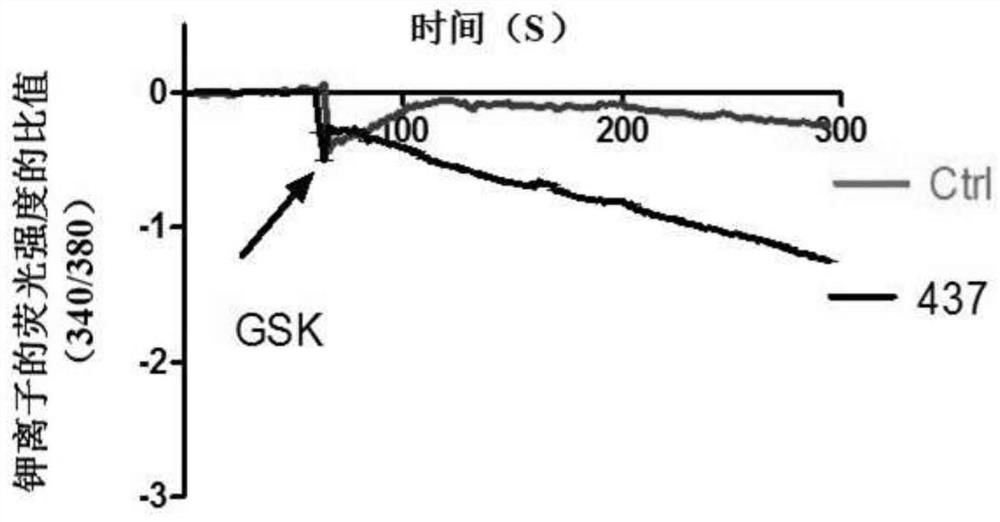
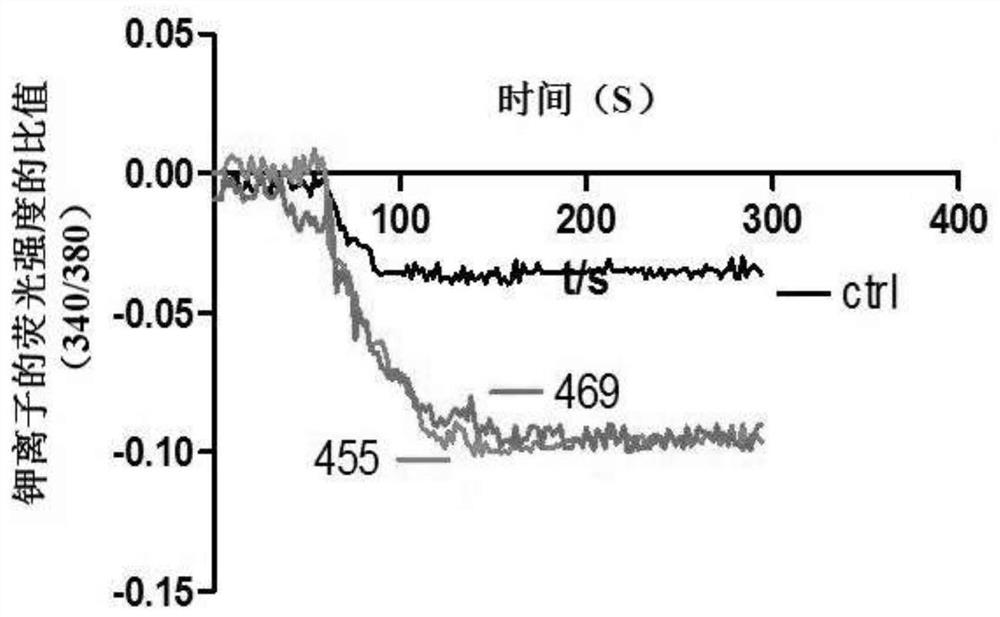
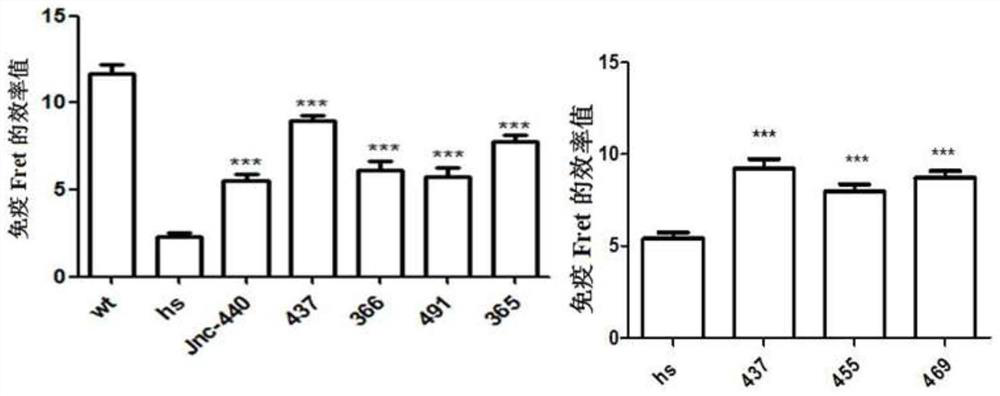
Compound for specifically enhancing spatial coupling degree of TRPV4-KCa2.3 complex and application of compound
A compound and composition technology, applied in the field of chemical medicine, can solve problems such as many side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: 4-oxo-N-(3-(2-(pyridin-4-yl)acetamido)propyl)-1,4-dihydroquinazoline-2-carboxamide
[0066]
[0067] The synthesis method is as follows:
[0068]
[0069] Step a: Preparation of ethyl 4-oxo-1,4-dihydroquinazoline-2-carboxylate
[0070] Suspend 2-aminobenzamide (10g, 73.53mmol) in diethyl oxalate (100mL), slowly heat to 185°C, reflux for 5 hours, after cooling to room temperature, a solid precipitates, filter, and filter cake petroleum ether Washed and dried to obtain 13 g of white solid with a yield of 86%.
[0071] Step b: Preparation of 4-oxo-1,4-dihydroquinazoline-2-carboxylic acid
[0072] 4-Oxo-1,4-dihydroquinazoline-2-carboxylic acid ethyl ester (2g, 9.2mmol, 1.0eq) was suspended in tetrahydrofuran (20mL) and water (10mL), and potassium hydroxide ( 1.5g, 27.5mmol, 3.0eq), stirred at room temperature for 2 hours. The ethanol was removed by rotary evaporation, the reaction liquid was cooled to 0°C, and the pH was adjusted to 2-3 with dilute hydr...
Embodiment 2
[0080] Example 2: 4-oxo-N-(3-(3-(pyridin-4-yl)ureido)propyl)-1,4-dihydroquinazoline-2-carboxamide
[0081]
[0082] The synthesis method is as follows:
[0083]
[0084] Step f: Preparation of phenylpyridin-4-ylcarbamate
[0085] Dissolve 4-aminopyridine (200mg, 2.13mmol, 1.0eq) in DMF (2mL), add triethylamine (257.9mg, 2.55mmol, 1.2eq), cool to 0°C, and slowly add phenyl chloroformate ( 399mg, 2.55mmol, 1.2eq), react at 0°C for 3 hours. The reaction solution was diluted with ethyl acetate, washed three times with water, washed once with saturated sodium chloride, dried over anhydrous sodium sulfate, concentrated to obtain 365 mg, yield 82%
[0086] Step g: Preparation of 4-oxo-N-(3-(3-(pyridin-4-yl)ureido)propyl)-1,4-dihydroquinazoline-2-carboxamide
[0087] N-(3-Aminopropyl)-4-oxo-1,4-dihydroquinazoline-2-carboxamide hydrochloride (100 mg, 0.353 mmol, 1.0 eq) was dissolved in DMF (2 mL), Add triethylamine (107.2mg, 1.06mmol, 3.0eq) and phenylpyridin-4-ylcarbamate (...
Embodiment 3
[0089] Example 3: N-(3-(3-(4-(((4-ethylpiperazin-1-yl)methyl)phenyl)ureido)propyl)-4-oxo-1,4 -Dihydroquinazoline-2-carboxamide
[0090]
[0091] The synthesis method is as follows:
[0092]
[0093] Step h: Preparation of phenyl (4-((4-ethylpiperazin-1-yl)methyl)phenyl)carbamate
[0094] 4-(4-Ethylpiperazine-1-methyl)-aniline (500mg, 2.28mmol, 1.0eq) was dissolved in DMF (5mL), triethylamine (345.9mg, 3.42mmol, 1.5eq) was added, Cool down to 0°C, add phenyl chloroformate (391.2mg, 2.51mmol, 1.1eq) dropwise, react at room temperature for 2h after addition, dilute the reaction solution with ethyl acetate, wash with water, wash with saturated sodium chloride, and dry over anhydrous sodium sulfate , concentrated, and column chromatography gave 480 mg of oily substance, with a yield of 62%.
[0095] Step i: N-(3-(3-(4-(((4-ethylpiperazin-1-yl)methyl)phenyl)ureido)propyl)-4-oxo-1,4- Preparation of dihydroquinazoline-2-carboxamide
[0096] N-(3-Aminopropyl)-4-oxo-1,4-dihydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com