Trpv4 antagonists
A technology selected from, alkyl, applied in the field of TRPV4 antagonists, can solve problems such as increasing left ventricular end-diastolic pressure and pulmonary artery blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
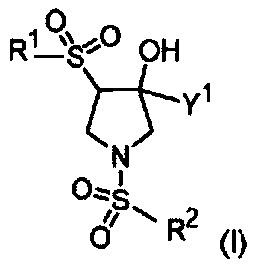
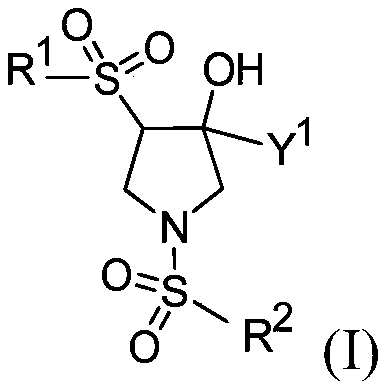
[0760] 3-Chloro-4-(((3R,4S)-4-((5-chloropyridin-2-yl)sulfonyl)-3-hydroxy-3-(hydroxymethyl)pyrrolidine- 1-yl)sulfonyl)benzonitrile
[0761]
[0762] step 1: (R)-3-((5-Chloropyridin-2-yl)thio)-4-methylenepyrrolidine-1-carboxylic acid tert-butyl ester
[0763]
[0764] Dissolve (S)-tert-butyl 3-methylene-4-((methylsulfonyl)oxy)pyrrolidine-1-carboxylate (22.2 g, 80 mmol) in DMF (300 mL) and add 5 -Chloropyridine-2-thiol (11.7g, 80mmol), then add K 2 CO 3 (16.7 g, 120 mmol). The reaction mixture thickened with stirring and was complete after 1 hour at room temperature. The mixture was poured into ice water, and extracted with 400 mL ethyl acetate / hexane (1:1 V / V). The organic extracts were washed with water (2x 400 mL), washed with MgSO 4 Dry, filter, and concentrate to give crude product. Purified by flash column chromatography (SiO 2 ), eluting with a gradient of 0-15% EtOAc / hexanes. The combined product fractions were concentrated to afford the title compound...
Embodiment 2
[0774] 3-Chloro-4-(((3R,4S)-4-((4-chlorophenyl)sulfonyl)-3-hydroxy-3-(hydroxymethyl)pyrrolidin-1-yl) Sulfonyl)benzonitrile
[0775]
[0776] step 1: (R)-3-((4-Chlorophenyl)sulfanyl)-4-methylenepyrrolidine-1-carboxylic acid tert-butyl ester
[0777]
[0778] To a solution of (S)-tert-butyl 3-methylene-4-((methylsulfonyl)oxy)pyrrolidine-1-carboxylate (8.85 g, 31.9 mmol) in DMF (177 mL) was added K 2 CO 3 (8.82g, 63.8mmol), then 4-chlorothiophenol (5.54g, 38.3mmol) was added, and the reaction mixture was stirred at room temperature for 16 hours, then diluted with water and extracted with EtOAc (4 times). The combined organic layers were concentrated under reduced pressure, and the crude product was subjected to flash column chromatography (SiO 2 ) was purified, eluting with a gradient of 0-40% MTBE / hexane. The desired fractions were combined, concentrated under reduced pressure and dried under high vacuum to afford the title compound as a white solid (5.14 g, 49% y...
Embodiment 22
[0798] 4-(((3S,4R)-1-((2-bromo-4-fluorophenyl)sulfonyl)-4-hydroxy-4-(hydroxymethyl)pyrrolidin-3-yl) Sulfonyl)benzonitrile
[0799]
[0800] step 1: (R)-3-((4-cyanophenyl)thio)-4-methylenepyrrolidine-1-carboxylic acid tert-butyl ester
[0801]
[0802] To a 3-necked flask equipped with a mechanical stirrer and a thermocouple was added tert-butyl (S)-3-methylene-4-((methylsulfonyl)oxy)pyrrolidine-1-carboxylate (28.8 g, 104 mmol) in DMF (300 mL). Add 4-mercaptobenzonitrile (16.9g, 125mmol), then add K 2 CO 3 (21.5 g, 156 mmol), and the reaction mixture was stirred at room temperature for 1 hour. Additional DMF (100 mL) was added to facilitate stirring, and two additional portions of 4-mercaptobenzonitrile (4.2 g each, 31 mmoL) were added 30 min apart. H 2 Quenched with O (500 mL) and extracted with hexane / EtOAc (1:1, 2x500 mL). The combined extracts were washed with H 2 O (4x 500mL), brine (1x500mL), washed with Na 2 SO 4 Dry, filter and concentrate under red...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com