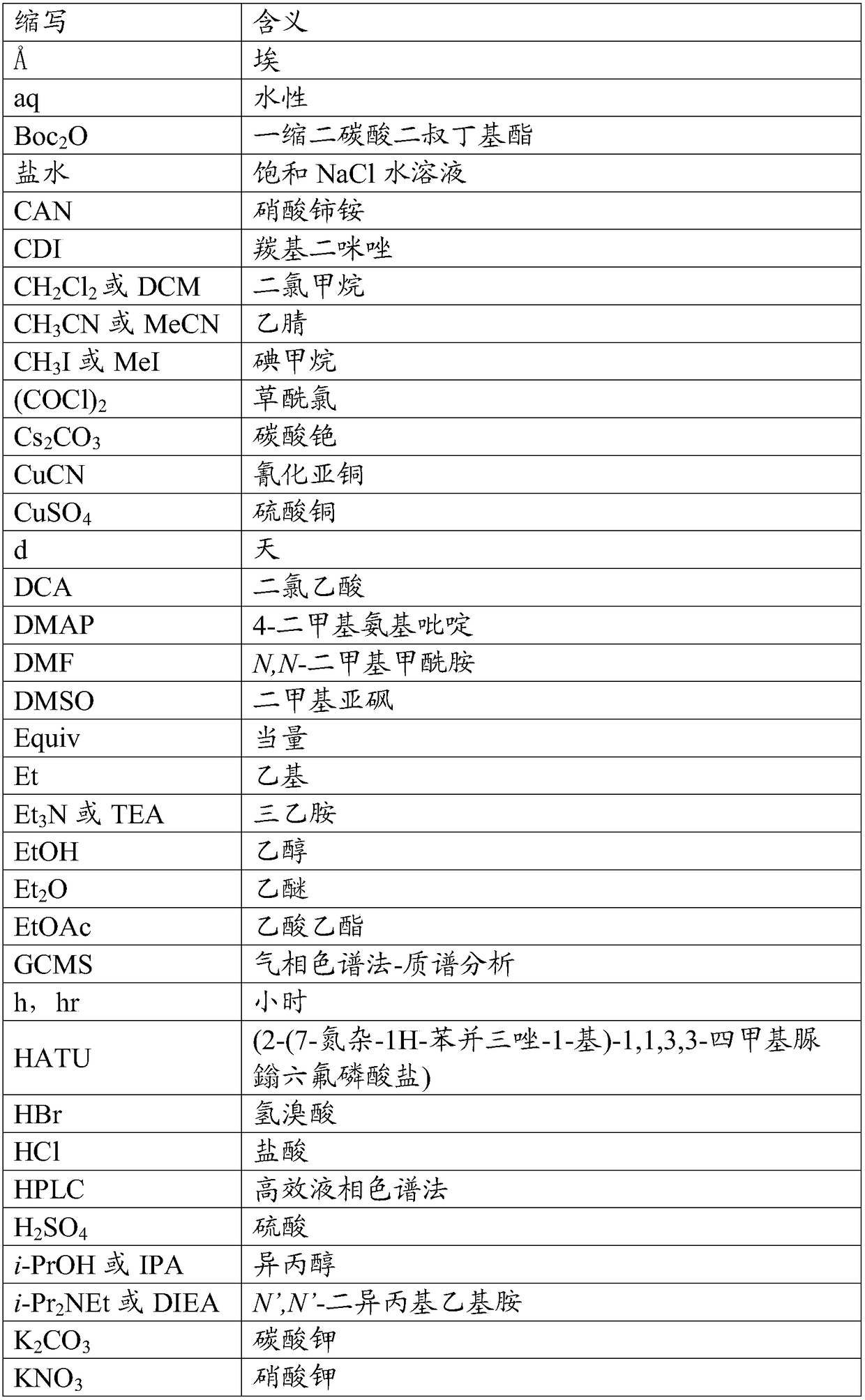
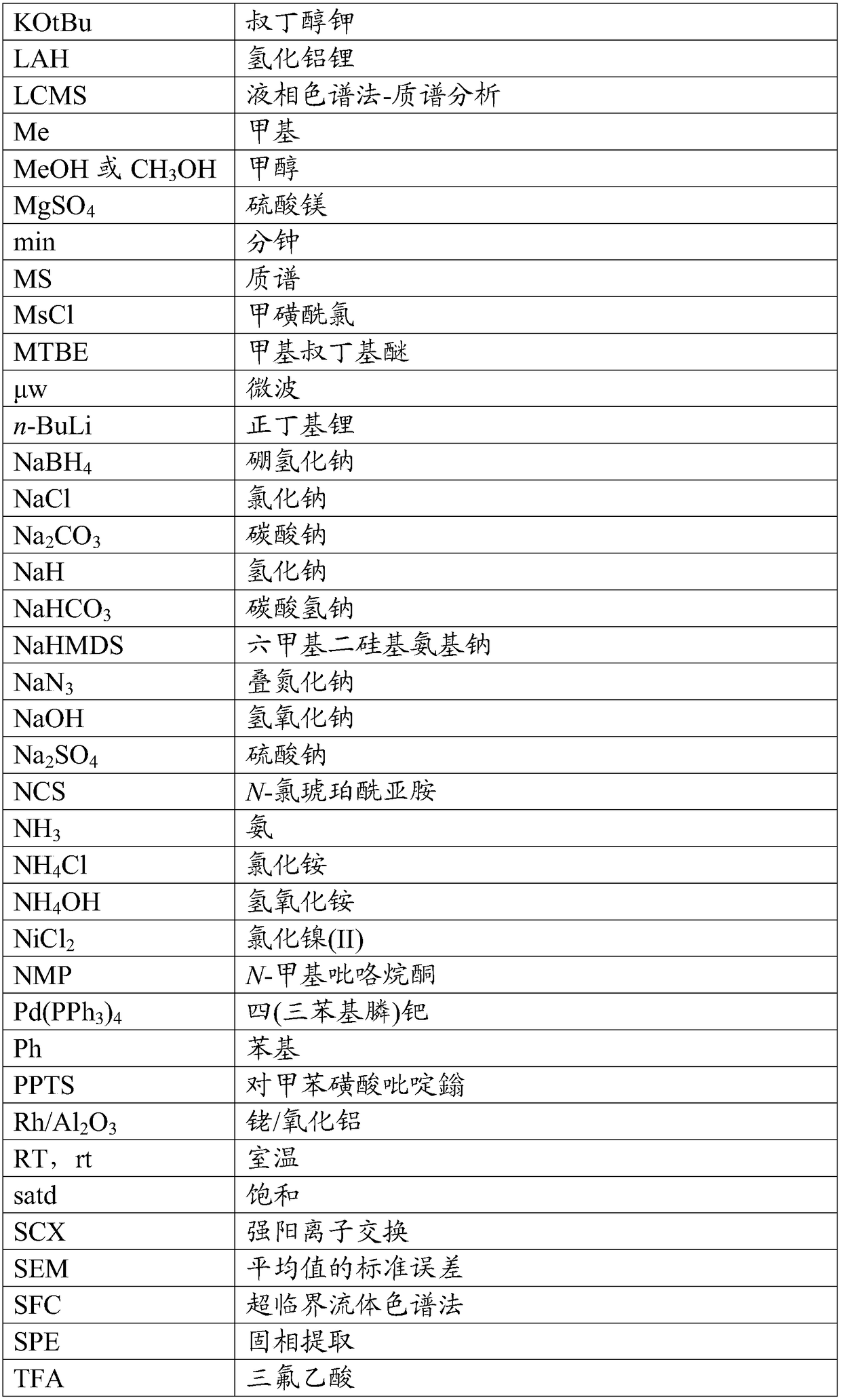
Trpv4 antagonist
A compound and pharmaceutical technology, applied in anti-inflammatory agents, anti-bacterial drugs, anti-tumor drugs, etc., can solve the problem of decreased ability of the left ventricle to pump blood into the peripheral circulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
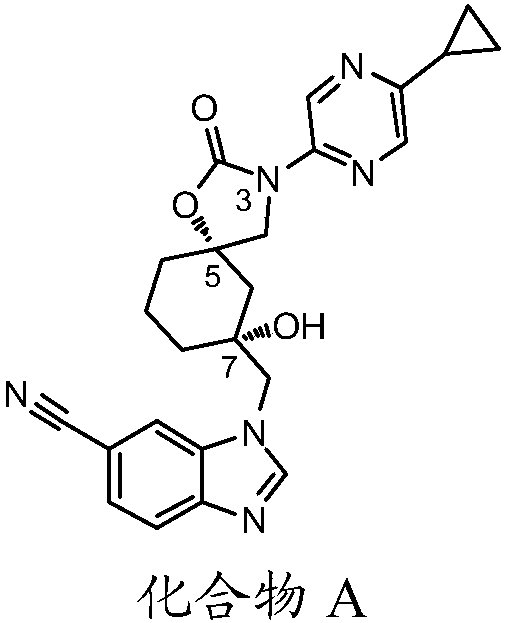
[0081] Preparation: 1-(((5S,7R)-3-(5-cyclopropylpyrazin-2-yl)-7-hydroxy-2-oxo-1-oxa-3-azaspiro[4.5] Decane-7-yl)methyl)-1H-benzo[d]imidazole-6-carbonitrile (compound A)
[0082]
[0083] plan 1
[0084]
[0085] Scenario 2
[0086]
[0087] Option 3
[0088]
[0089] Phase 1: 1,4-dioxaspiro[4.5]decane-7-one
[0090] Cyclohexane-1,3-dione (500g, 4459mmol), A solution of molecular sieves (500 g, 4459 mmol) and p-toluenesulfonic acid (254 g, 1338 mmol) in anhydrous ethylene glycol (2 L) was stirred at room temperature under nitrogen for 4 hours. The reaction mixture was washed with saturated NaHCO 3 The solution (1 L) was diluted to adjust to basic pH, and the basic mixture was extracted with ethyl acetate (3 X 1 L). The combined organic extracts were washed with brine solution (500ml) and washed with Na 2 SO 4 Dry, filter, and concentrate under reduced pressure to give the title compound (280 g, 1732 mmol, 38.8% yield) as a yellow liquid. LCMS (m / z) 1...
Embodiment 2
[0113] Example 2 - Capsule Composition
[0114] Oral dosage forms for administration of the present invention are prepared by filling standard two-part hard gelatin capsules with the ingredients and proportions shown in Table 1 below.
[0115] Table 1
[0116]
Embodiment 3
[0117] Example 3 - Injectable Parenteral Compositions
[0118] The injectable form for administration of the present invention is prepared by adding 1.7% by weight of 1-(((5S,7R)-3-(5-cyclopropylpyrazin-2-yl)-7-hydroxy-2-oxy Generation-1-oxa-3-azaspiro[4.5]decane-7-yl)methyl)-1H-benzo[d]imidazole-6-carbonitrile (compound A) in 10% by volume of propylene glycol aqueous solution Prepared by stirring.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com