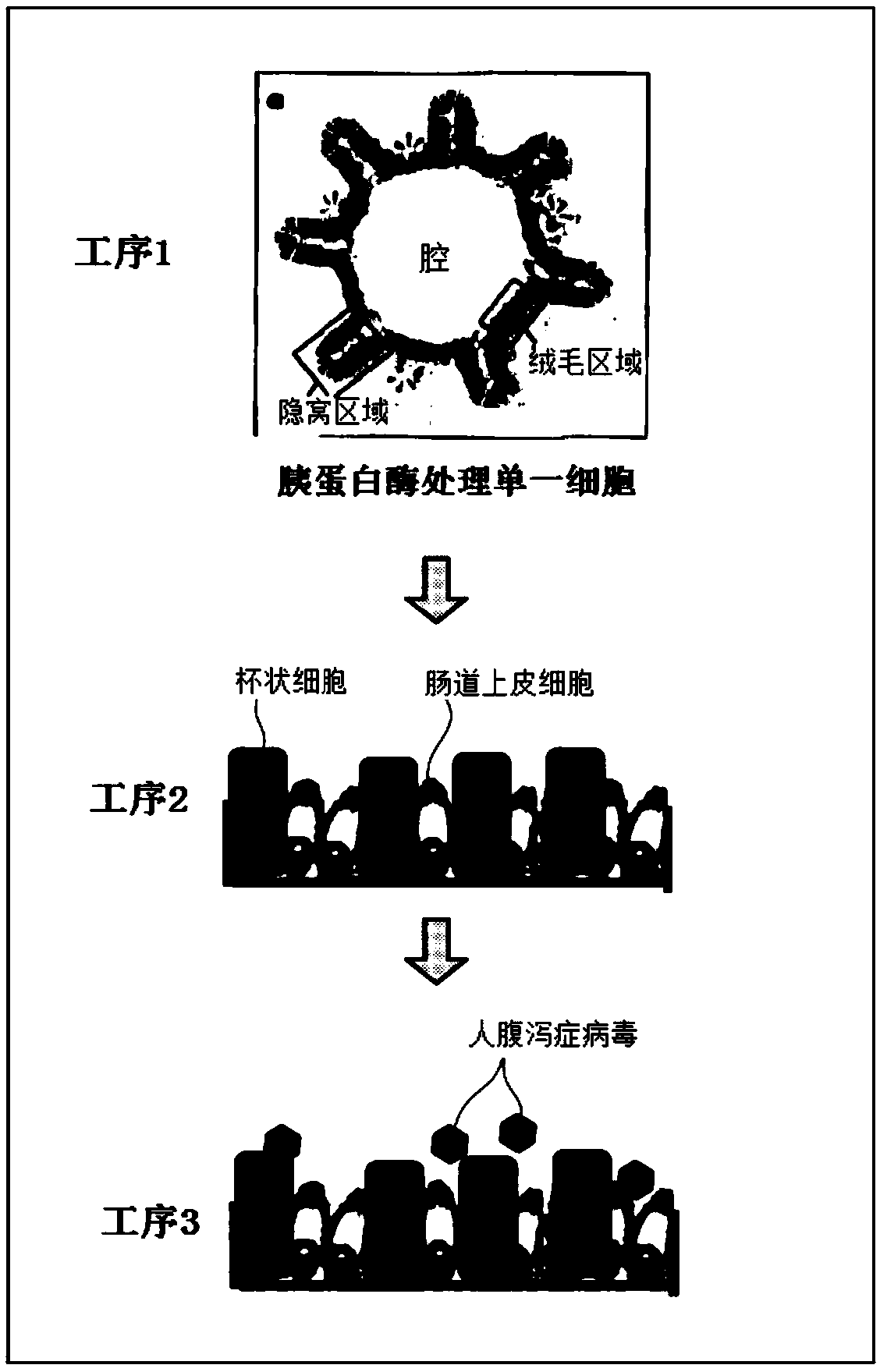
2d organoid for infection and culture of human diarrhea virus, and use of said 2d organoid
A technology for proliferation culture and organoids, which can be applied in the direction of virus, 3D culture, culture process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0146] Examples of methods for producing ECM include methods using bound tissue cells, and the like. More specifically, after culturing ECM-producing cells such as fibroblasts, removing these cells and adding epithelial stem cells, epithelial cells, epithelial tumor cells, or tissues containing them, ECM can be used as a base site.
[0147] Examples of ECM-producing cells include chondrocytes that mainly produce collagen and proteoglycans, fibroblasts that mainly produce type IV collagen, laminin, interstitial procollagen, and fibronectin, and that mainly produce collagen (type I, type III and type V), chondroitin sulfate proteoglycan, hyaluronic acid, fibronectin and tenascin-C colonic myofibroblasts, etc. Alternatively, commercially available ECMs can also be used. Examples of commercially available ECMs include: extracellular matrix proteins (manufactured by Invitrogen), basement membrane preparations derived from Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells (for exampl...
experiment example 1
[0188] (Preparation of cell culture medium)
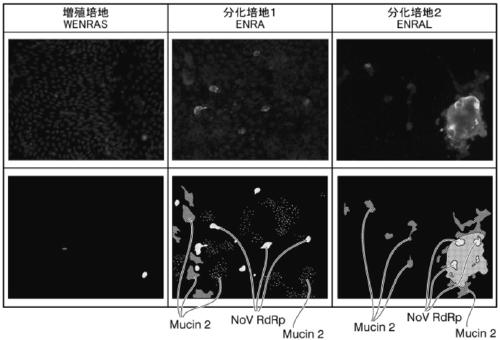
[0189] First, to commercially available Advanced DMEM / F-12 medium (manufactured by ThermoFicher SCIENTIFIC), human recombinant R-spondin 1 (manufactured by R&D systems) was added to a final concentration of 1 μg / mL, and noggin ( Peprotech) to a final concentration of 100 ng / mL, A83-01 (Tocris) was added to a final concentration of 500 nM (hereinafter referred to as "NRA medium").
[0190] Further, a culture medium was prepared in which the following combinations were added: Wnt3a at a final concentration of 300 ng / mL, IGF1 at a final concentration of 500 ng / mL (manufactured by Biolegend), FGF2 at a final concentration of 50 ng / mL (peprotech company), EGF (manufactured by Thermo Ficher SCIENTIFIC) at a final concentration of 50 ng / mL, SB202190 (manufactured by Sigma Aldrich) at a final concentration of 10 μM, and LY411575 (manufactured by Sigma Aldrich) at a final concentration of 1 μM.
[0191] ·WNRA+IGF1+FGF2 Medium
[0192] ·WE...
experiment example 2
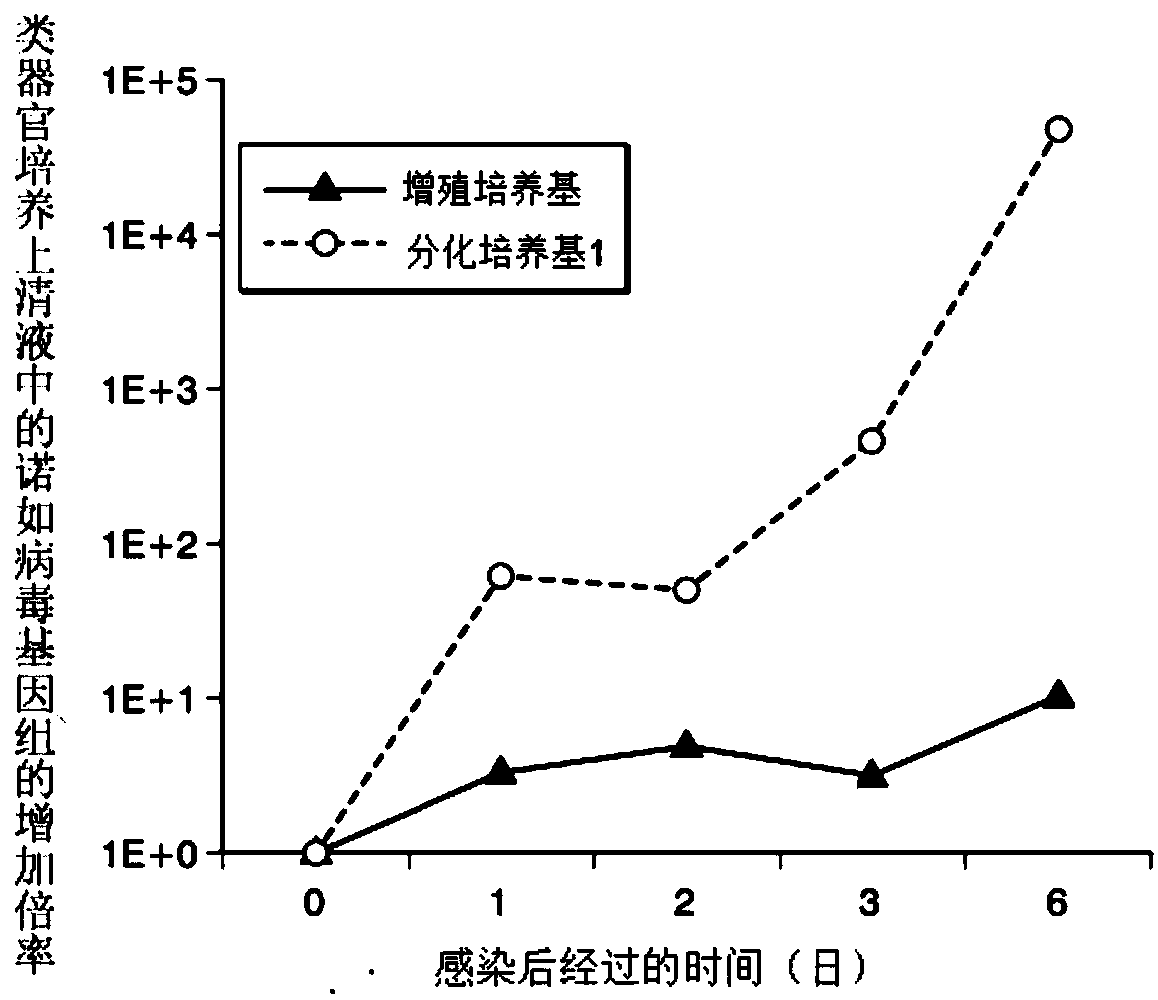
[0203] (Proliferation of Norovirus Using Organoids)
[0204] "Cultivation of Intestinal Stem Cells"
[0205] Based on an ethical research plan approved by the Ethics Committee of the Faculty of Medicine, Keio University, normal mucosa was collected at least 5 cm away from the gastrointestinal tumor from healthy subjects and patients with gastrointestinal tumors who had obtained instructions and consent. Epithelial cells were extracted from the collected tissue by means of EDTA or Liberase TH, and embedded in Matrigel (registered trademark).
[0206] Matrigel (registered trademark) containing epithelial cells (hereinafter referred to as "intestinal stem cells") was seeded on a 48-well plate and cultured. Specifically, as follows.
[0207] The cultured intestinal stem cells were seeded on a 48-well plate together with 25 μL of Matrigel (registered trademark) (manufactured by BD Bioscience). 100 µL per well of the WNRA+IGF1+FGF2 medium prepared in (1) was added to each well, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com