Preparation method of high-purity propofol
A technology of propofol and purity, applied in the field of preparation of high-purity propofol, can solve problems such as equipment corrosion, achieve the effects of simple process, simplified preparation process and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
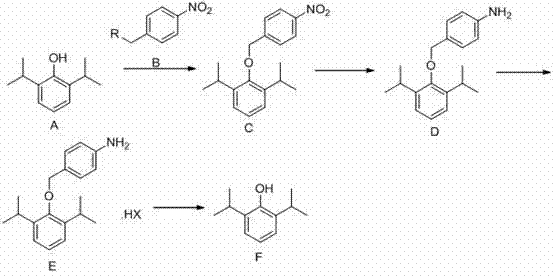
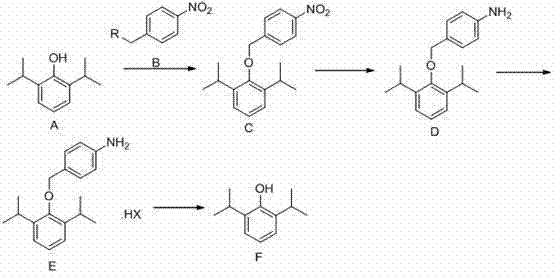
[0042] Example 1: 2,6-diisopropylphenol (178g; 1mol) was dissolved in 200ml of DMF (N,N'-dimethylformamide), keeping the temperature below 25°C, adding potassium carbonate (152g , 1.1mol), after stirring for half an hour, p-nitrobenzyl bromide (238g, 1.1mol) was added dropwise, and the temperature was controlled not to exceed 25°C. After the dropwise addition, the reaction was incubated for 4 hours. After the reaction, add 600ml of water, extract three times with 300ml of dichloromethane, wash the dichloromethane layer once with 200ml of saturated brine, dry over anhydrous sodium sulfate, and concentrate to dryness under reduced pressure to obtain 266.38g of oil, with a yield of 85%.
[0043] Dissolve the oil in 2000ml of ethanol, add ferric chloride (25.93g, 0.16mol) and activated carbon (25.20g, 2.10mol), stir and heat up to 80°C, slowly add 80% hydrazine hydrate (311ml, 5.1 mol), after dropping, keep it warm for 2 hours, filter while hot, and concentrate the filtrate to dr...
Embodiment 2
[0047]Dissolve 2,6-diisopropylphenol (178g; 1mol) in 200ml of acetone, keep the temperature below 25°C, add potassium carbonate (152g, 1.1mol) in batches, stir for half an hour, then add p-nitrobenzyl dropwise Bromine (238g, 1.1mol), control the temperature not to exceed 25°C. After the dropwise addition, the reaction was incubated for 4 hours. After the reaction, add 600ml of water, extract three times with 300ml of dichloromethane, wash the dichloromethane layer once with 200ml of saturated brine, dry over anhydrous sodium sulfate, and concentrate to dryness under reduced pressure to obtain 266.38g of oil, with a yield of 85%.
[0048] Dissolve the oil in 2000ml of ethanol, add stannous chloride dihydrate (959g, 4.23mol), and react at 70°C for 4h. After the reaction, cool to room temperature, concentrate to dryness, add 500ml of water, adjust the pH to above 11 with 10% aqueous sodium hydroxide solution, extract three times with 300ml of ethyl acetate, dry over anhydrous so...
Embodiment 3
[0052] 2,6-Diisopropylphenol (89g; 0.5mol) was dissolved in 100ml tetrahydrofuran, keeping the temperature below 25°C, adding sodium carbonate (58.3g, 0.55mol) in batches, stirring for half an hour, then adding p-nitrogen dropwise Benzyl benzyl bromide (119g, 0.55mol), control the temperature not to exceed 25°C. After the dropwise addition, the reaction was incubated for 4 hours. After the reaction, add 300ml of water, extract three times with 150ml of dichloromethane, wash the dichloromethane layer once with 100ml of saturated brine, dry over anhydrous sodium sulfate, and concentrate to dryness under reduced pressure to obtain 120g of oil, with a yield of 76.5%.
[0053] Dissolve the oil in 1000ml of ethanol, add ferric chloride (11.67g, 0.07mol) and activated carbon (11.34g, 0.95mol), stir and heat up to 80°C, slowly add 80% hydrazine hydrate (140ml, 2.30 mol), after dropping, keep it warm for 2 hours, filter while hot, and concentrate the filtrate to dryness to obtain 94.9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com