Preparation method for bromhexine hydrochloride
A technology of bromhexine hydrochloride and bromhexine, applied in the field of preparation of bromhexine hydrochloride, which can solve the problems of large environmental pollution, harsh reaction, and high cost of intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The invention provides a kind of preparation method of bromhexine hydrochloride, comprising the following steps:
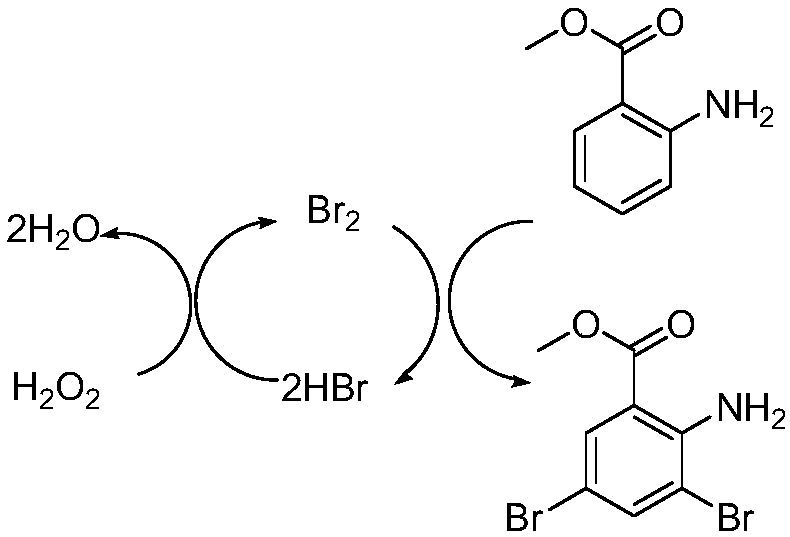
[0056] (1) carry out bromination reaction after mixing 2-aminobenzoic acid ester compound, bromine, oxidizing agent and mixed solvent, generate 3,5-dibromo-2-aminobenzoic acid ester;
[0057] (2) Mixing the obtained 3,5-dibromo-2-aminobenzoic acid ester, a catalyst, a reducing agent and a solvent to perform a reduction reaction to generate 3,5-dibromo-2-aminobenzyl alcohol;
[0058] (3) carry out condensation reaction after the gained 3,5-dibromo-2-aminobenzyl alcohol, N-methylcyclohexylamine, mixed catalyst and solvent are mixed, generate bromhexine;
[0059] (4) Gained bromhexine and hydrochloric acid are carried out salt-forming reaction in solvent, generate bromhexine hydrochloride.
[0060] The invention mixes 2-aminobenzoic acid ester compounds, bromine, oxidizing agent and mixed solvent to perform bromination reaction to generate 3,5-dibromo-2-amino...
Embodiment 1
[0102] The raw materials in this example are all commercially available.
[0103] Synthesis of methyl 3,5-dibromo-2-aminobenzoate:
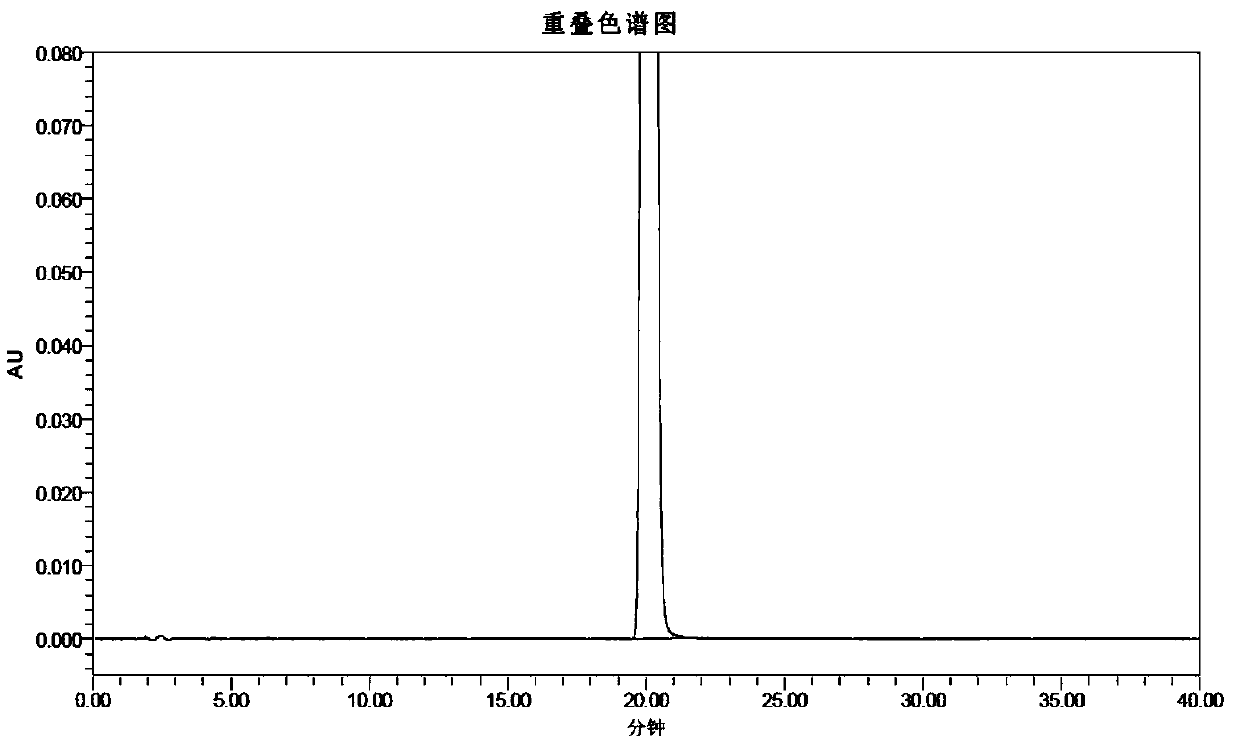
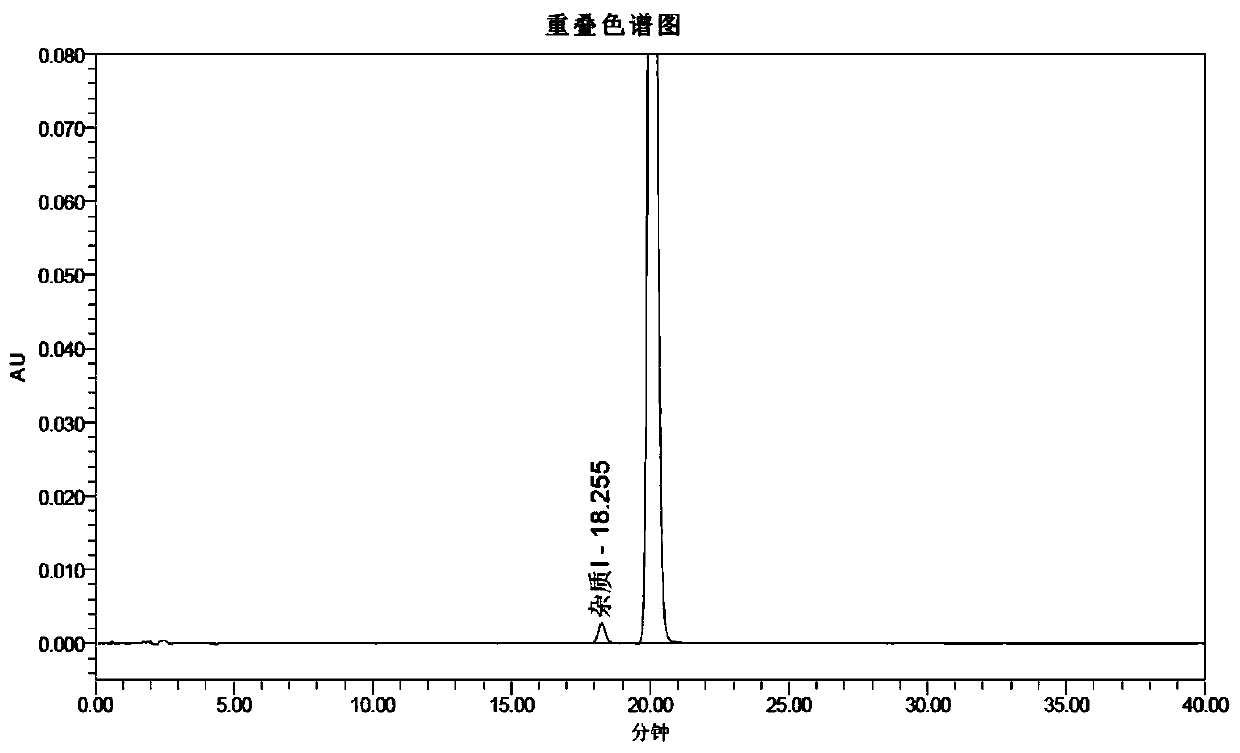
[0104] 26.50 kg of dichloromethane, 8.0 kg of purified water, and 3.02 kg of methyl 2-aminobenzoate were successively added into a 100L double-layer glass reactor, and stirred evenly. At room temperature, a solution of bromine (3.83 kg) in dichloromethane (5.3 kg) was slowly added to the reaction solution obtained above. Continue to stir for 10 minutes after the addition is complete, slowly add 2.50 kg of 30% hydrogen peroxide solution to the reaction solution, stir for 6 hours after the reaction is complete, slowly add 3.5 kg of 20% sodium sulfite solution to the reaction solution to quench the reaction, and separate phases , the organic phase was extracted once with 8.0 kg of purified water, the aqueous phase was extracted twice with dichloromethane, the organic phases were combined, the organic phase was concentrated under reduced pressure un...
Embodiment 2
[0136] The 3.02kg 2-aminobenzoic acid methyl ester among the embodiment 1 is replaced by 3.3kg 2-aminobenzoic acid ethyl ester, other conditions are exactly the same, finally bromhexine hydrochloride can be successfully prepared, and the yields of each step are equivalent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com