Preparation method of levamlodipine besylate
A technology of levamlodipine besylate and amlodipine is applied in the field of pharmaceutical synthesis and can solve the problems of difficult green treatment of mother liquor, no industrial application prospect, low production cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
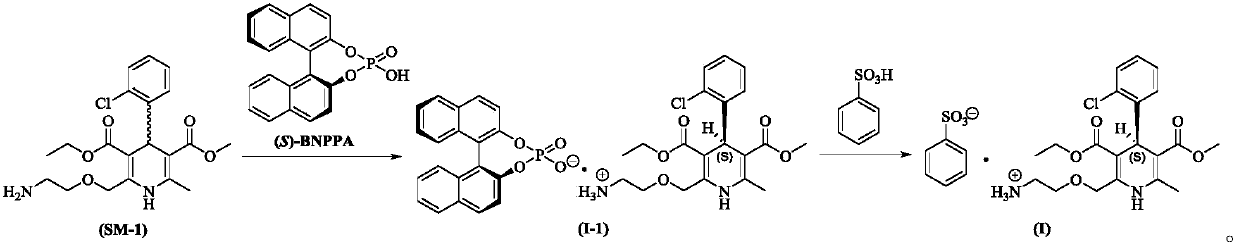
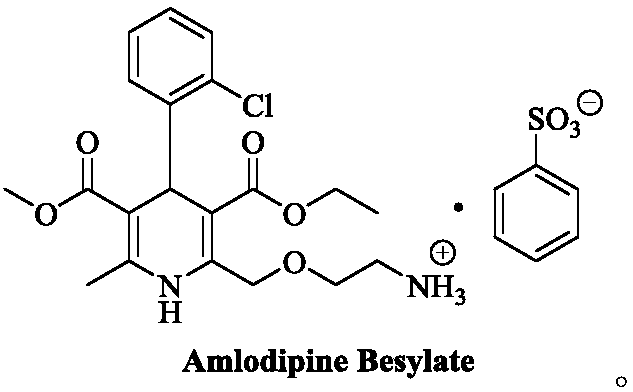
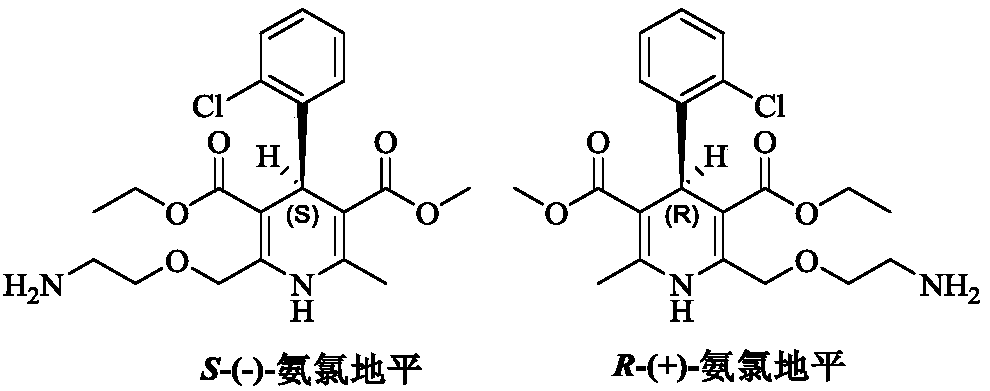
[0066] At room temperature, add (R,S)-amlodipine (20.44g, 0.05mol) into methanol (150mL), stir until dissolved, then add (S)-BNPPA (11.32g, 32.5mmol), and control the temperature at 30~ After the material is completely dissolved at 35°C, the temperature of the reaction solution is controlled at 0-5°C to crystallize for 7 hours. After the crystallization is completed, filter with suction, rinse the filter cake with methanol (50mL×2), and add the obtained filter cake to methanol-purified aqueous solution (V 水 :V 醇 =8:1, 200mL), add benzenesulfonic acid (4.11g, 26.0mmol) in purified water (20mL) at a temperature of 30-35°C. After the crystallization, suction filtration, filter cake purified water (50mL × 2) rinse, the resulting filter cake was dried under reduced pressure to obtain 13.1g of levamlodipine besylate, the molar yield was 46.2%, and the purity was detected by HPLC , where t R =12.023min is levamlodipine besylate, the purity is 99.89%, and no impurity D is detected;...
Embodiment 2
[0068] At room temperature, add (R,S)-amlodipine (20.44g, 0.05mol) into methanol (140mL), stir until dissolved, then add (S)-BNPPA (9.75g, 28.0mmol), and control the temperature for 25~ After the material is completely dissolved at 30°C, the temperature of the reaction solution is controlled at -5 to 0°C to crystallize for 7 hours. After the crystallization is completed, filter with suction, rinse the filter cake with methanol (50mL×2), and add the obtained filter cake to methanol-purified aqueous solution (V 水 :V 醇 =10:1, 200mL), add benzenesulfonic acid (4.11g, 26.0mmol) in purified water (20mL) solution at temperature control 30-35°C. After the crystallization is over, suction filtration, filter cake purified water (50mL × 2) rinse, the resulting filter cake after drying under reduced pressure is 12.5g of levamlodipine besylate, the molar yield is 44.1%, through HPLC purity detection , where t R =12.025min is levamlodipine besylate, the purity is 99.85%, and impurity D i...
Embodiment 3
[0070] At room temperature, add (R,S)-amlodipine (20.44g, 0.05mol) into methanol (140mL), stir until dissolved, then add (S)-BNPPA (9.40g, 27.0mmol), and control the temperature for 25~ After the material is completely dissolved at 30°C, the temperature of the reaction solution is controlled at -5 to 0°C to crystallize for 7 hours. After the crystallization is completed, filter with suction, rinse the filter cake with methanol (50mL×2), and add the obtained filter cake to methanol-purified aqueous solution (V 水 :V 醇 =10:1, 200mL), add benzenesulfonic acid (4.11g, 26.0mmol) in purified water (20mL) solution at 30-35°C temperature control, after the dropwise addition, turn to salt and crystallize at 20-25°C temperature control for 10h, After the crystallization, suction filtration, filter cake purified water (50mL * 2) rinse, the obtained filter cake is 12.0g of levamlodipine besylate after drying under reduced pressure, the molar yield is 42.3%, through HPLC purity detection , ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com