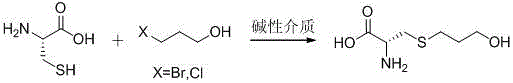Preparation method of fodor stanozolol suitable for industrialized production
A technology for fodosteine and amine compounds, applied in the field of medicine, can solve the problems of product loss, cumbersome steps, long reaction time, etc., and achieve the effects of improving quality and simplifying process
Active Publication Date: 2016-07-20
迪嘉药业集团股份有限公司
View PDF7 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0009] The biggest disadvantage of this method is that it is difficult to remove the inorganic salts in the final product, which easily leads to high residue
For example, US5047428 has reported that L-cysteine and 3-bromo-1-propanol react to prepare fudosteine. The method has a long reaction time, and the reaction process can produce equimolar inorganic salts, and fudosteine is soluble in water. , insoluble in organic solvents, it is not easy to remove inorganic salts from the product, resulting in high product residues, which is not conducive to industrial production; Chinese patent application 200910167947.0 reported the reaction of L-cysteine and 3-chloro-1-propanol to prepare Fodos Fudosteine, using the solubility difference between fudosteine and sodium chloride to purify fudosteine, but the experimental process requires hot filtration (≥95°C), the steps are cumbersome, the operation is complicated, and the product will be more lost
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0020] Example 2
Embodiment 2
[0022] Example 3
Embodiment 3
[0024] Example 4
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a preparation method of fodor stanozolol suitable for industrialized production. According to the preparation method disclosed by the invention, water-ethanol is used as a reaction solvent, amine substances are used as a catalyst, after the reaction is completed, temperature reduction and crystallization are performed, and products are separated in a precipitation form. The whole process is easy to control and high in repeatability, the yield of the fodor stanozolol is stabilized to 95%, and the residue quantity is controlled within 0.05%, so that medical standards are completely met, and the preparation method is very suitable for industrialized production.
Description
technical field [0001] The invention relates to the preparation of fudosteine, an antitussive and phlegm-reducing drug, and belongs to the technical field of medicine. Background technique [0002] Fudosteine (chemical name: 3-hydroxypropylthioalanine) is a class of compounds with the basic skeleton of steine developed by Japan SSP Pharmaceutical Co., Ltd. in 1988. It was first introduced in October 2001 Listed in Japan. Pharmacological experiments have proved that it has many functions such as inhibiting goblet cell hyperplasia, promoting serous tracheal secretion and inhibiting tracheal inflammation. Due to its strong efficacy, small side effects, wide indications and great market potential, fudosteine is expected to become Alternative products of similar drugs. [0003] The synthetic method of this compound is mainly divided into two classes at present: [0004] One method is the reaction of L-cysteine and allyl alcohol under the action of light, microwave, hea...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C319/18C07C323/58
CPCC07C319/18C07C323/58
Inventor 李廷义蒋增强葛执信苗华明
Owner 迪嘉药业集团股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com