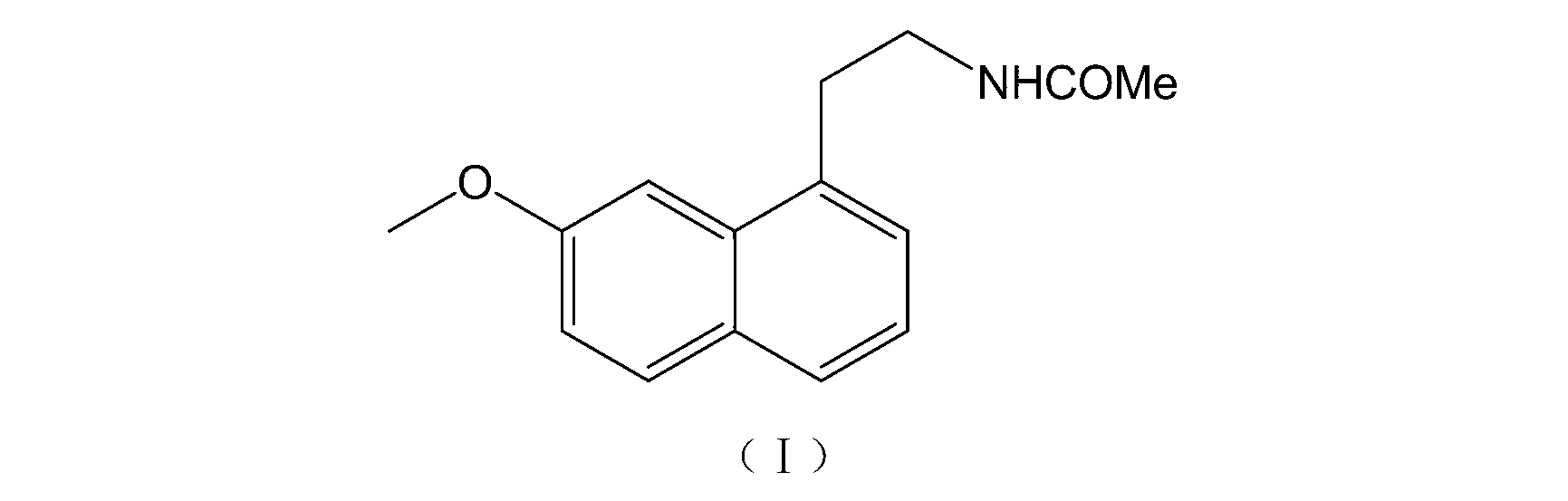
Novel agomelatine crystal form L and preparation method thereof
A technology of agomelatine crystal and L type, which is applied in the field of medicinal chemical synthesis, can solve the problems of process stability, poor repeatability, harsh preparation conditions, and is difficult to large-scale industrial production, and achieves remarkable medicinal effect and operation. Simple, high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
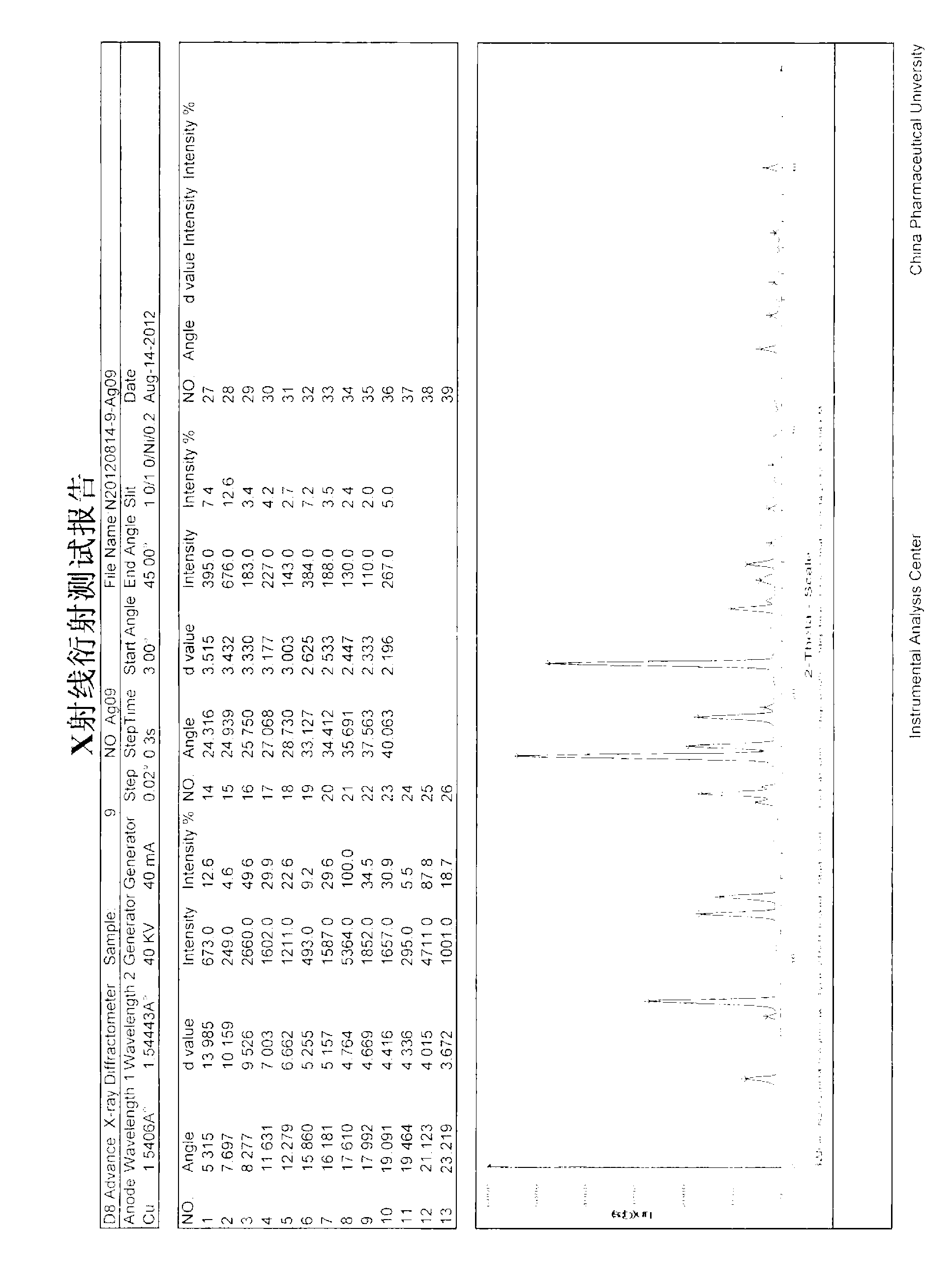
[0034] Take 1g of agomelatine (purity 99.5%) in a 100ml reaction bottle, add 10ml of toluene and stir at room temperature, it does not dissolve, heat to 80°C, still not completely dissolved, then add another 10ml of toluene, the solid dissolves completely. Stop heating, open to cool naturally, precipitate crystals, until the toluene is completely volatilized, transfer to 40°C for vacuum drying. Obtained 0.98g solid with a yield of 98%, a purity of 99.58%, and a residual solvent of toluene of 0.015% (according to the requirements of the 2010 edition of the Chinese Pharmacopoeia, the residual toluene solvent of the raw material should be ≤0.089%), melting point: 107.3-108.8°C, and X-ray powder diffraction pattern see attached figure 1 .
Embodiment 2
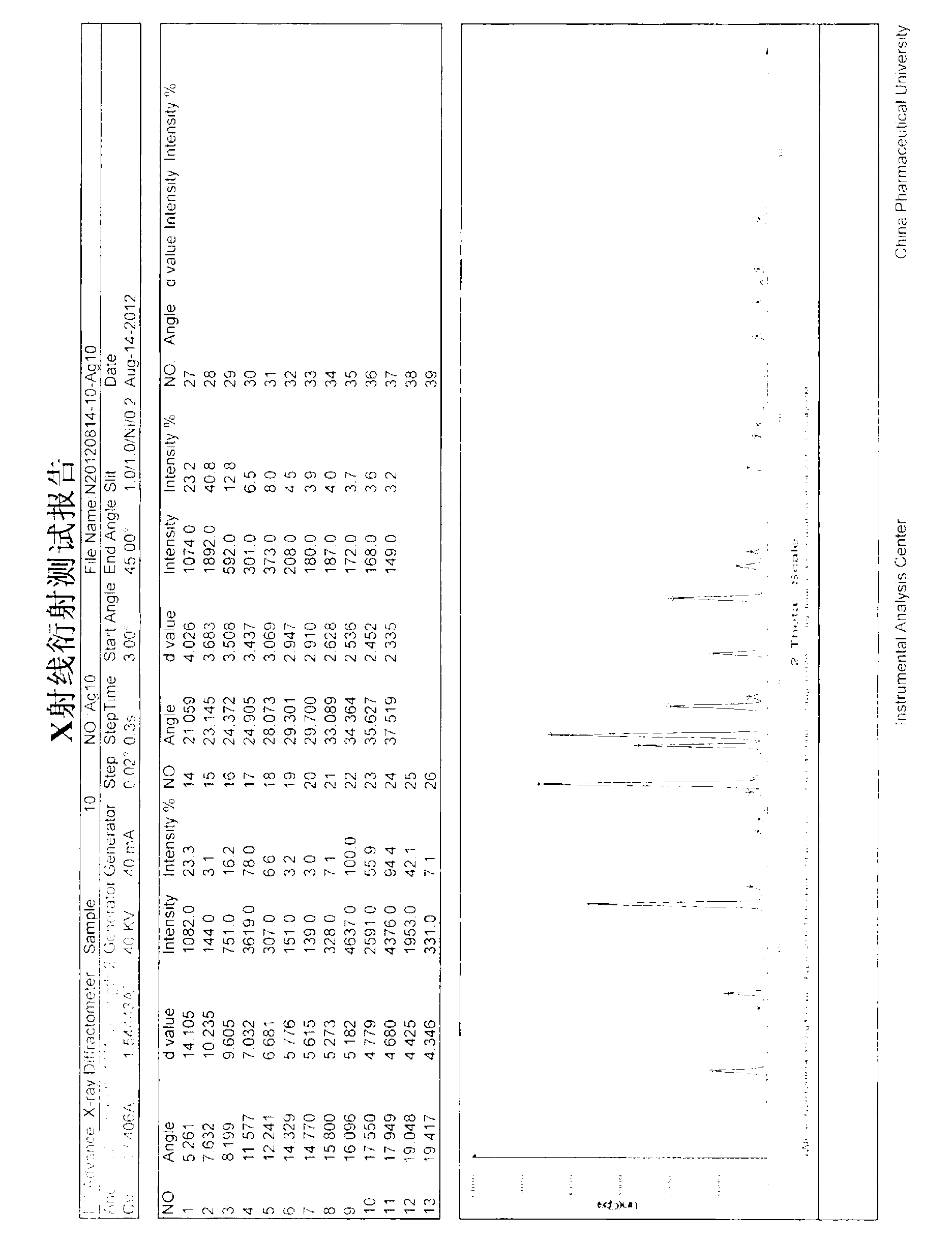
[0036] Take 1g of agomelatine (purity 99.5%) in a 100ml reaction bottle, add 20ml of isopropyl ether and stir at room temperature, it does not dissolve, heat to 80°C, still not completely dissolved, then add 20ml of isopropyl ether, the solid is completely dissolve. Stop heating, open to cool naturally, precipitate crystals, until the isopropyl ether volatilizes completely, transfer to 40°C for vacuum drying. Obtained 0.99g solid, the yield was 99%, the purity was 99.55%, and the residual solvent of isopropyl ether was 0.12% (according to the requirements of the Chinese Pharmacopoeia 2010 edition, the residual solvent of isopropyl ether in raw materials should be ≤0.5%), melting point: 107.5- 108.7°C, its X-ray powder diffraction pattern is shown in the attached figure 2 .
Embodiment 3
[0038]Take 5g of agomelatine (purity 99.5%) in a 250ml reaction bottle, add 100ml of toluene and stir at room temperature, if it does not dissolve, heat it to 80°C, and the solid will dissolve completely. Stop heating, open to cool naturally, precipitate crystals, until the toluene is completely volatilized, transfer to 40°C for vacuum drying. Obtained 4.96g of solid, the yield was 99.2%, the purity was 99.51%, the residual solvent of toluene was 0.018% (according to the requirements of the Chinese Pharmacopoeia 2010 edition, the residual toluene solvent of the raw material drug should be ≤0.089%), the melting point: 107.2-108.6 ° C, its X-ray powder diffraction pattern see attached figure 1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com