Preparation and purifying method of tiagabine hydrochloride
A tiagabine hydrochloride and purification method technology, applied in the direction of organic chemistry, can solve the problems of laborious, time-consuming column chromatography operation, high cost, etc., and achieve the effect of lightening the color, reducing the cost and improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
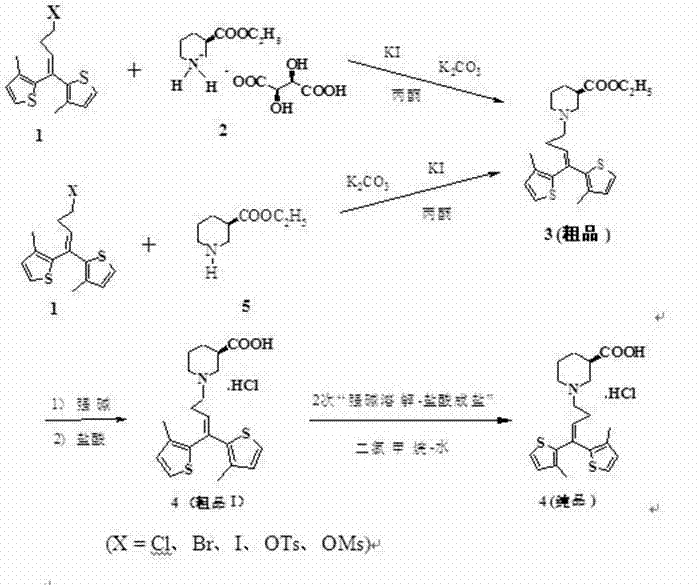
[0031] Embodiment 1: via 1,1-bis(3-methyl-2-thienyl)-4-bromo-1-butene (1) and R -Piperidine-3-ethyl carboxylate (5) to prepare tiagabine hydrochloride (control group for yield and purity)
[0032] R -Ethyl piperidine-3-carboxylate 20.4g (130mmol), anhydrous potassium carbonate 62.9g (456mmol), potassium iodide 1.08g, 1.1-bis(3-methyl-2-thienyl)-4-bromo-1- A mixture of 42.1 g (130 mmol) of butene and 600 ml of acetone was stirred electromagnetically for 72 hours at room temperature in the dark, filtered and washed, and the filtrate was concentrated to obtain an orange-red oil. The crude product was subjected to column chromatography [ f 45×300, silica gel 200-300 mesh, elution: petroleum ether→petroleum ether-acetone (20:1)] to obtain 39.7g of a light yellow oil in the distillate, with a yield of 76.0%.
[0033] The product of the previous step was dissolved in 200ml of 95% ethanol, 51ml of 4M LiOH solution was added, and stirred at room temperature for 3 hours. Under ic...
Embodiment 2
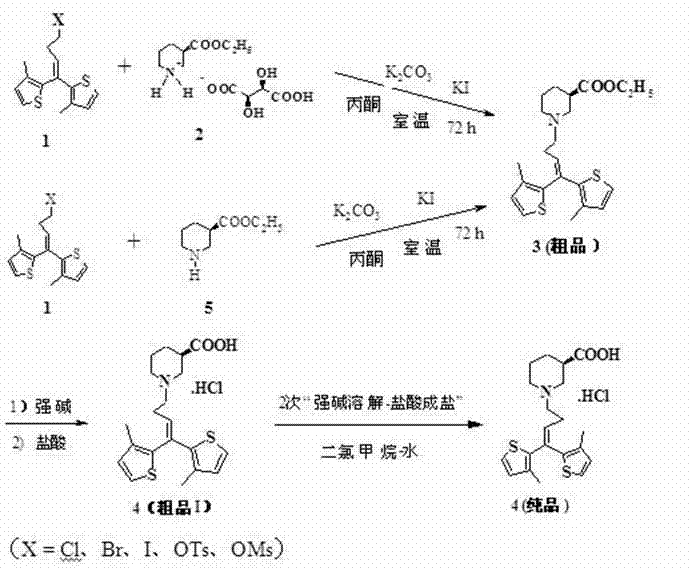
[0035] Embodiment 2: the yield verification of alkylation reaction——( R )-Piperidine-3-ethyl carboxylate. L-tartrate is directly charged, and the reaction of 1,1-bis(3-methyl-2-thienyl)-4-bromo-1-butene prepares tiagabine pure ethyl ester
[0036] ( R )-piperidine-3-carboxylic acid ethyl ester. L-tartrate 42.0g (137mmol), 1,1-bis(3-methyl-2-thienyl)-4-bromo-1-butene 44.7g (137mmol ), anhydrous K 2 CO 3 66.1 g (479 mmol), KI 1.16 g and acetone 630 ml, the reactant was protected from light, stirred at room temperature for 72 hours, filtered to remove inorganic salts, and the filtrate was rotary evaporated (bath temperature <50°C). The filter residue was washed three times with acetone, and the filtrate was rotary evaporated (bath temperature <50°C) to obtain a red oil, which was separated by column chromatography (silica gel, eluent: petroleum ether→petroleum ether-acetone) to obtain 43.7 g of a light yellow product, which was collected The rate is 77.5%.
[0037] ...
Embodiment 3
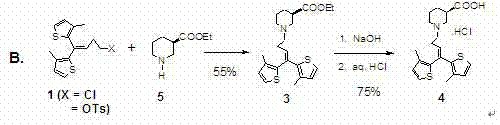
[0038] Embodiment 3: the yield verification of alkylation reaction——( R )-Piperidine-3-ethyl carboxylate. L-tartrate is fed directly and prepared by reaction with 1,1-bis(3-methyl-2-thienyl)-1-butene-4-methanesulfonate Pure Tiagabine Ethyl Ethyl
[0039] at room temperature R- Piperidine-3-carboxylic acid ethyl ester×L-tartrate 34.1g (111mmol), 1,1-bis(3-methyl-2-thienyl)-4-methanesulfonate-1-butene 40.1g ( 111mmol), anhydrous K 2 CO 3 A mixture of 53.6g (388mmol), KI 0.93g and acetone 550mL was stirred for 72 hours, filtered to remove inorganic salts, the filtrate was rotary evaporated, and the residue was purified by column chromatography (silica gel 300~400 mesh, petroleum ether:acetone=95:5) 33.7 g of yellow oil was obtained, with a yield of 75.5%, which was close to the reported yield of 74-77%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com