Fludarabine phosphate preparation method
A technology of fludarabine phosphate and triethyl phosphate, which is applied in the field of synthesis of antimetabolite and antitumor drugs, can solve the problems of low yield and the purity of the product cannot meet the pharmaceutical standards, and achieves high yield and easy separation. Purification and the effect of small residual organic solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Add 20g of fludarabine to a 500mL flask, then add 200mL of triethyl phosphate, put the flask into a low-temperature reaction bath at -6°C, and slowly add 20mL of phosphorus oxychloride dropwise after 20 minutes (stir while adding) , reacted for 12 hours (TCL tracking). After the reaction meets the requirements, quickly add 80mL water and 200mL dichloroethane to the flask, let it stand for 30 minutes, extract the aqueous phase and the organic phase, and adjust the pH value of the aqueous phase to 2-3. Recrystallization appears white floc with a cloth Filtered through a funnel, vacuum dried and weighed to obtain 20.71g of white powder, yield 81.02%, HPLC mass fraction 99.95%, elemental analysis results are all mass fractions (C 33 h 30 N 4 o 2 ) Theoretical value (measured value): C 32.81 (32.89), H 3.65 (3.59), N 19.14 (19.17); ESI m / z (%, M-1): 364.2. 1 HNMR(DMSO-D6,400MHz)δ(ppm): 1 HNMR (DMSO-D6) δ3.94(2H, m), 4.09(2H, m), 4.152(1H, t, J=9.2Hz), 5.80(2H, d), 6.15(...
Embodiment 2
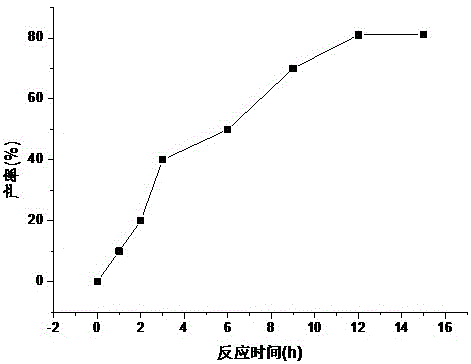
[0015] When synthesizing fludarabine phosphate, other conditions are fixed and only the reaction time is changed. This embodiment examines the influence of reaction time on the yield of fludarabine phosphate. figure 1 ,Depend on figure 1 It can be seen that when the reaction time is short, the yield is low; and when the reaction time is long, the yield does not increase much, the by-products increase, and the product mass fraction decreases (the HPLC mass fraction is only 96.1%). The optimal reaction time should be controlled within 12 hours, the reaction yield is high, the product mass fraction is high (the HPLC mass fraction of the refined product is 99.95%) and it is more economical.
Embodiment 3
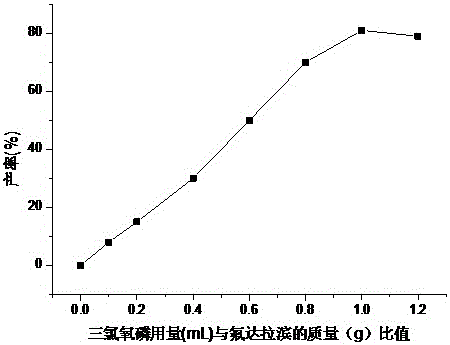
[0017] When synthesizing fludarabine phosphate, fix other conditions unchanged, only change the amount of phosphorus oxychloride, the present embodiment has investigated the influence of the amount of phosphorus oxychloride on the yield of fludarabine phosphate see figure 2 ,Depend on figure 2 It can be seen that with the increase of the amount of phosphorus oxychloride, the yield gradually increases; and when the amount of phosphorus oxychloride is too much, the yield of fludarabine phosphate decreases, the by-products increase, and the product mass fraction decreases. The optimal dosage of phosphorus oxychloride is 20mL, and the dosage of fludarabine is 20g. At this time, the reaction yield is high, the product mass fraction is high and it is more economical.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com