Preparation method of amino intermediate
An intermediate and amine-based technology, which is applied in the field of preparation of amine-based intermediates, can solve problems such as difficult removal, impact on nintedanib quality and drug safety, and unfavorable industrial production of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
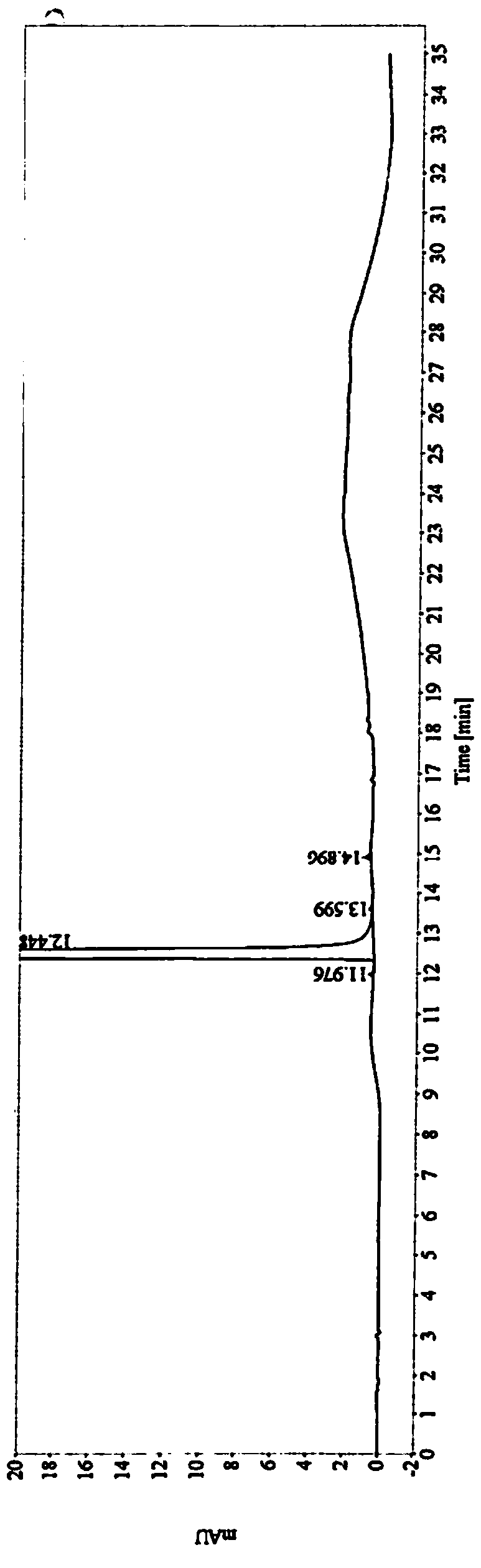
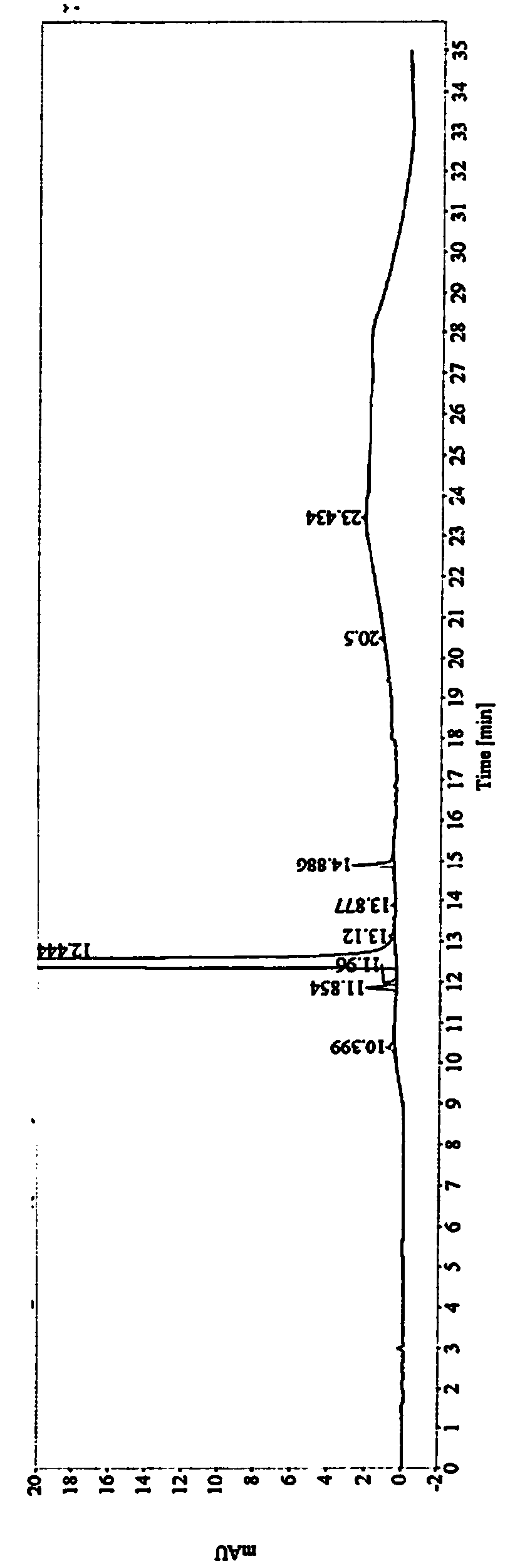
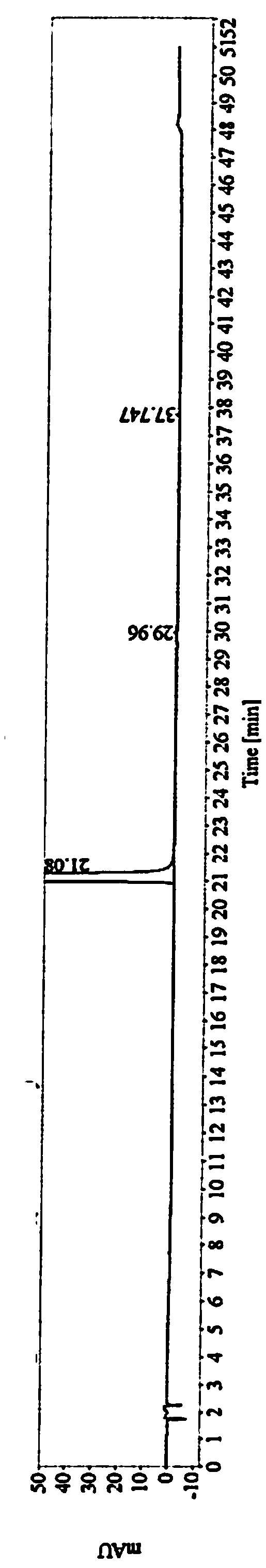
Image
Examples
Embodiment 1
[0023] Embodiment 1: the investigation of N-methylpiperazine adding mode in compound II synthesis
[0024] Add the compound of formula (III) into toluene, heat to 45°C, add N-methylpiperazine, stir and react at 50-60°C for 2 hours after the addition, cool to room temperature, add water, stir well, and then stand to separate the water layer . After the organic phase was diluted with isopropanol, palladium carbon was added for reduction. After the reaction was completed, it was filtered, concentrated and dried for inspection. The research results are as follows:
[0025] The temperature of the reaction solution at the time of addition Adding method of N-methylpiperazine Dosage of N-methylpiperazine join time Impurity 1 content in compound I 45℃ one-time join* 50g 1 minute 0.05% 45℃ Slowly add N-methylpiperazine dropwise 50g 15 minutes 0.07% 45℃ Slowly add N-methylpiperazine dropwise 50g 30 minutes 0.09% 45℃ Slowly add N-met...
Embodiment 2
[0028] Embodiment 2: the investigation of reaction solution initial temperature in compound II synthesis
[0029] Add the compound of formula (III) into toluene, heat to different temperatures, add N-methylpiperazine at one time, after the reaction is completed, cool to room temperature and add water, stir evenly and then stand still to separate the water layer. The organic phase was diluted by adding isopropanol, and then reduced by adding palladium carbon. After the reaction was completed, it was filtered, concentrated and dried for inspection. The research results are as follows:
[0030] The temperature of the reaction solution at the time of addition Adding method of N-methylpiperazine Dosage of N-methylpiperazine experimental phenomenon Impurity 1 content in compound I 10℃ one-time join 50g up to 50°C 0.05% 30℃ one-time join 50g up to 60°C 0.03% 45℃ one-time join 50g up to 90°C 0.05% 80℃ one-time join 50g reflow ...
Embodiment 3
[0032] Embodiment 3: the investigation of compound I refining solvent
[0033] The obtained crude compound of formula (I) is added into a refined solvent, heated to dissolve and crystallized. The precipitated solid was air-dried at 60°C and then detected. The research results are as follows:
[0034]
[0035] Conclusion: Under the same refining operation, alcohol solvents have the best impurity removal effect. Among alcoholic solvents, isopropanol has the highest yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com