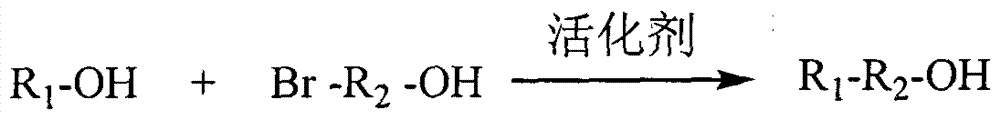Multifunctional synergistic pharmaceutical composition based on adriamycin and construction method of multifunctional synergistic pharmaceutical composition
A doxorubicin and multi-functional technology, applied in the field of pharmaceutical preparations and supramolecular chemistry, can solve the problems of limiting the optimization and application of hydrophobic small molecule compounds containing phenolic hydroxyl conjugates, poor activity of phenolic hydroxyl groups, etc., to prolong the circulation time in the body, Good uniformity, avoiding the effect of protease degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: the synthesis of curcumin-unfractionated heparin polymer
[0074] Weigh an appropriate amount of 6-bromohexylamine hydrochloride into an eggplant-shaped bottle, add dichloromethane to dissolve, and then add di-tert-butyl dicarbonate. The molar ratio of di-tert-butyl dicarbonate to 6-bromohexylamine hydrochloride is 1.5:1. Separately weigh 1-hydroxybenzotriazole and triethylamine, dissolve them in an appropriate amount of dichloromethane, and slowly drop them into the above-mentioned eggplant-shaped bottle. The molar ratio of 1-hydroxybenzotriazole to 6-bromohexylamine hydrochloride is 1:5, and the molar ratio of triethylamine to 6-bromohexylamine hydrobromide is 1.05:1. After reacting at room temperature for 40 min, wash with 0.5 mol / L dilute sulfuric acid, saturated sodium bicarbonate solution and saturated sodium chloride solution three times respectively. After washing, the organic phase was dried with anhydrous sodium sulfate for 2 h. Suction filtra...
Embodiment 2
[0078] Embodiment 2: the synthesis of curcumin-low molecular weight heparin polymer
[0079] Weigh an appropriate amount of curcumin and place it in an eggplant-shaped bottle, add an appropriate amount of acetone to dissolve, the molar concentration of curcumin is 0.05mmol / mL, add 1.1 molar amount of sodium carbonate, and then dropwise add 1.05 molar amount of 3-bromo-1- Propanol, heat and reflux at 60°C until the raw materials disappear. After the reaction is complete, filter while it is hot, drop the filtrate into a large amount of ice water, stand for crystallization, filter with suction, and dry to obtain the curcumin derivative intermediate with a free hydroxyl group at one end 1. Weigh an appropriate amount of low-molecular-weight heparin and dissolve it in formamide, heat and dissolve at 60°C for 2 hours, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and hydroxyl For succinimide activation, the molar ratio of low molecular weight heparin carboxyl to 1...
Embodiment 3
[0080] Embodiment 3: the synthesis of chrysin-low molecular weight heparin polymer
[0081] Weigh an appropriate amount of chrysin and put it in an eggplant-shaped bottle, add an appropriate amount of acetone to dissolve, the molar concentration of chrysin is 0.05mmol / mL, add 1.3 molar amount of potassium carbonate, and then dropwise add 1.1 molar amount of 3-bromo-1- Propanol, heated under reflux at 60°C until the raw materials disappear, after the reaction is complete, filter while hot, drop the filtrate into a large amount of ice water, let it stand for crystallization, filter with suction, and dry to obtain the intermediate of chrysin derivatives with a free hydroxyl group at one end 1. Weigh an appropriate amount of low-molecular-weight heparin and dissolve it in formamide, heat and dissolve at 60°C for 2 hours, protect it under nitrogen, and add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and hydroxyl For succinimide activation, the molar ratio of low mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com