Prodrug of oridonin with polyethylene glycol serving as vector and preparation method thereof
A technology of oridonin A and polyethylene glycol is applied in the field of natural medicine and polymer materials to achieve the effects of improving solubility, improving performance and improving performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
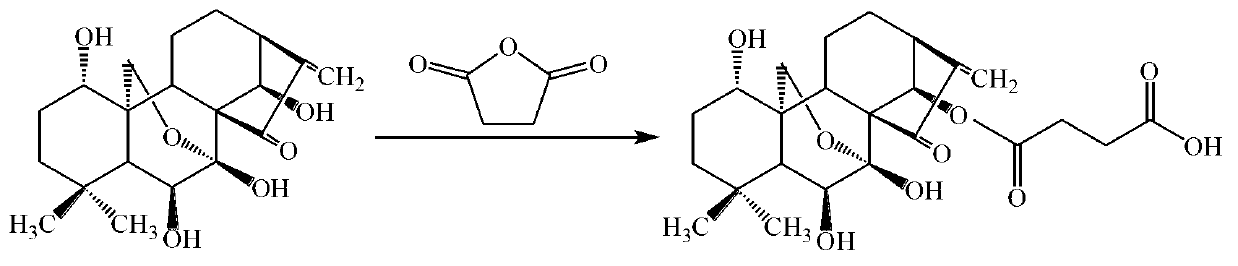
[0032] (1) Synthesis of carboxylated oridonin
[0033] Disperse and dissolve 0.6552g oridonin and 0.9006g succinic anhydride in 12mL dichloromethane. Add 3mL anhydrous pyridine to the system. The reaction temperature is 35℃. The reaction is magnetically stirred for 24 hours. The reaction solvent is concentrated and evaporated to dryness. Afterwards, the residue containing the reaction product was purified by a silica gel column (mobile phase: anhydrous ether: acetone: glacial acetic acid = 4.5:1:0.01, volume ratio; stationary phase: 200-300 mesh silica gel), and the solvent was evaporated after purification , The product is dried under vacuum to obtain a colorless oily succinic oridonin.
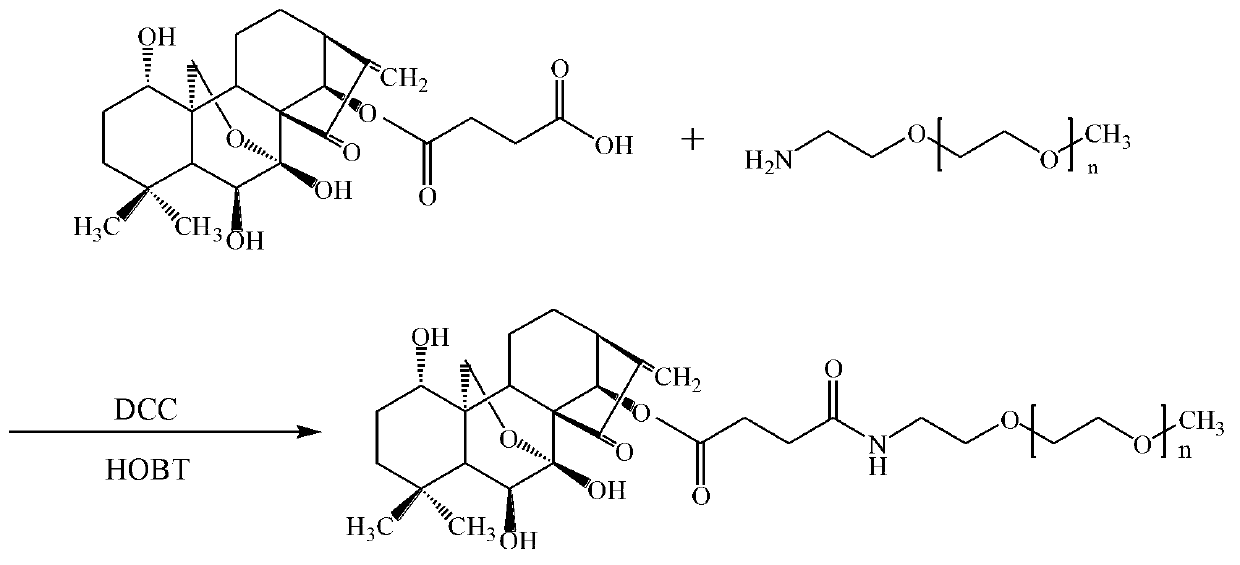
[0034] (2) Synthesis of oridonin prodrug modified by methoxy polyethylene glycol amine (40K)
[0035] Weigh out 0.1044g succinic oridonin, 0.0203g HOBT and 0.0309g DCC, disperse and dissolve them in 12mL dichloromethane, stir and react for 2h under an ice bath at 0~5℃, remove the ice bath and add ...
Embodiment 2
[0037] (1) Synthesis of carboxylated oridonin: the same as in Example 1.
[0038] (2) Synthesis of oridonin prodrug modified by methoxy polyethylene glycol amine (20K)
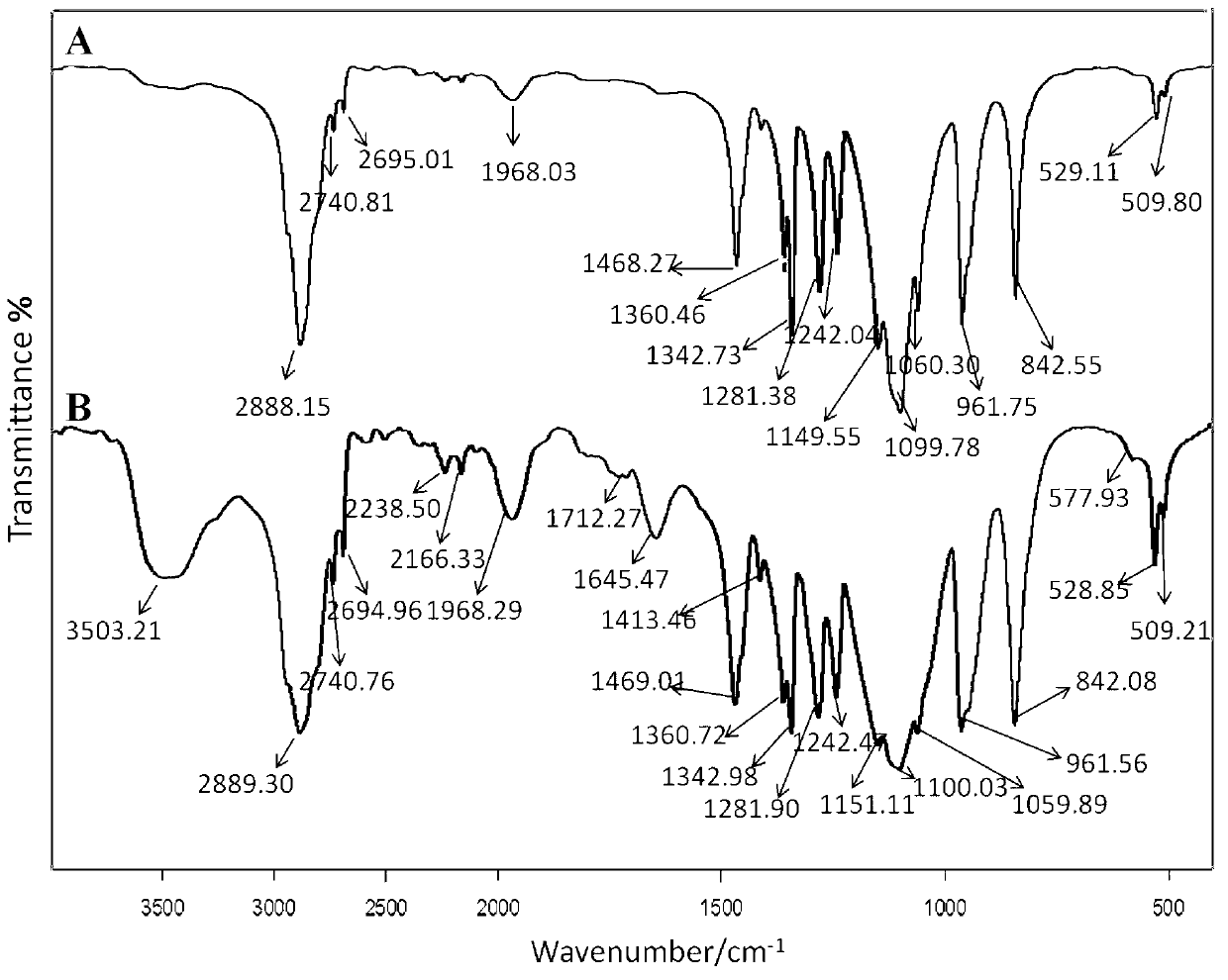
[0039] Weigh 0.1040g of succinic oridonin, 0.0207g HOBT and 0.0308g DCC, disperse and dissolve in 12mL dichloromethane, stir at 0~5℃ in an ice bath for 2h, remove the ice bath and add to the system 1.5g monomethoxypolyethylene glycol amine (20K), naturally warm to room temperature, continue the reaction for 48h, then concentrate and evaporate the solvent, the obtained solid is dispersed and stirred with isopropanol for 6h, so that unreacted small molecules are dissolved in In isopropanol, the small molecule impurities in the product are washed away. The isopropanol is washed three times, the supernatant is discarded by centrifugation, and the precipitate is vacuum dried to obtain a white solid product, which is methoxy polyethylene glycol amine (20K ) The infrared spectrum of the modified oridonin prodrug is as i...
Embodiment 3
[0044] (1) Synthesis of carboxylated oridonin: the same as in Example 1.
[0045] (2) Synthesis of oridonin prodrug modified by methoxy polyethylene glycol amine (10K)
[0046] Weigh 0.1392g of succinic oridonin, 0.0405g HOBT and 0.0619g DCC, disperse and dissolve in 15mL dichloromethane, stir and react for 2h in an ice bath at 0~5℃, remove the ice bath and add to the system 1.5g of methoxy polyethylene glycol amine (10K), naturally warm to room temperature, continue the reaction for 48h, then concentrate and evaporate the solvent, the obtained solid is dispersed and stirred with isopropanol for 6h, so that the unreacted small molecules are dissolved in the isopropyl alcohol In order to wash away the small molecule impurities in the product in propanol, wash three times with isopropanol, centrifuge to discard the supernatant, and vacuum dry the precipitate to obtain a white solid product, which is methoxy polyethylene glycol amine (10K) A modified oridonin prodrug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com