Preparation process and products of oridonin fine particles
A technology of oridonin A and fine particles is applied in the production of bulk chemicals, organic chemistry, drug combination and other directions, which can solve the problems of vascular inflammation and pain in patients, achieve convenient medication, reduce use costs and toxic side effects, and produce short cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
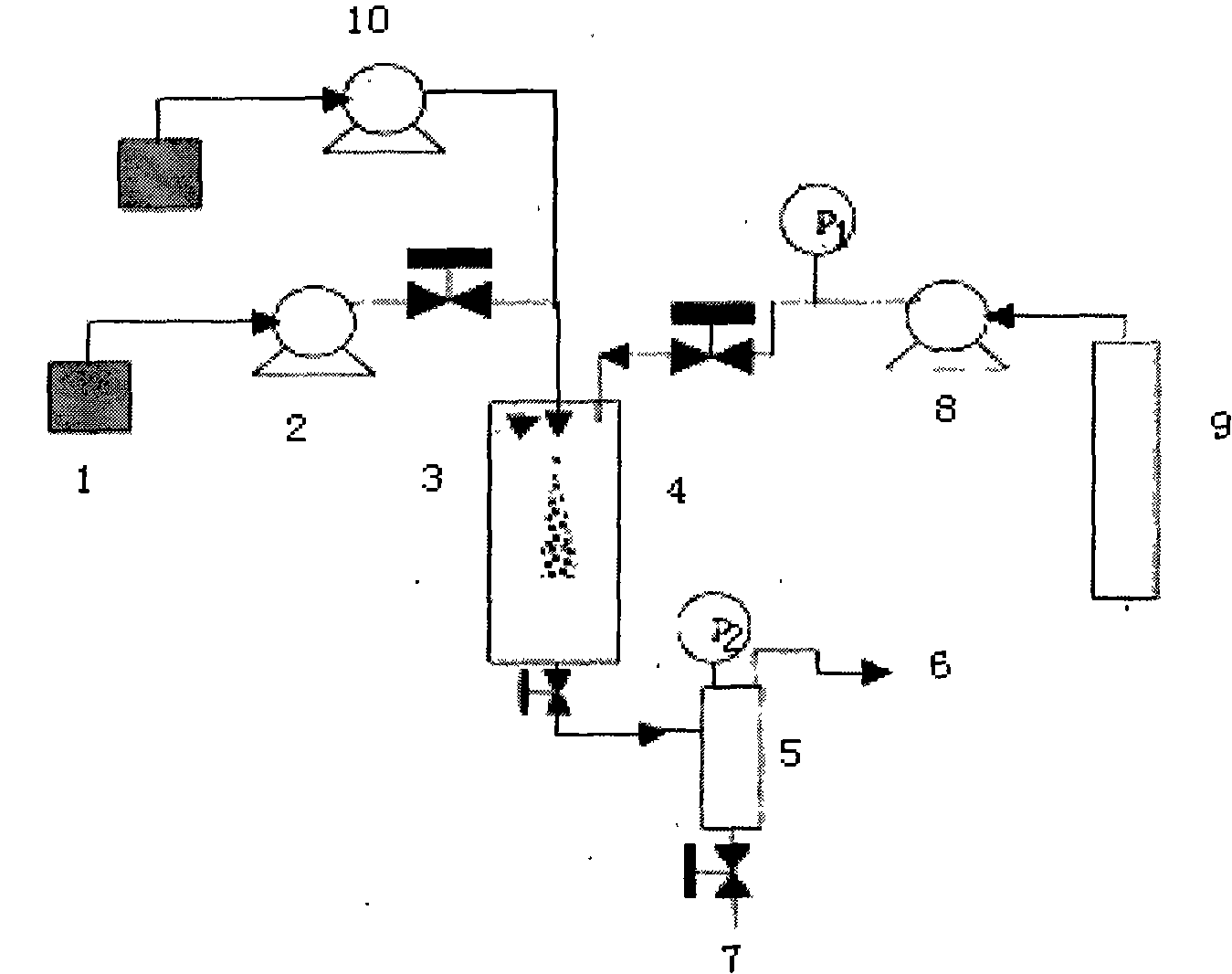
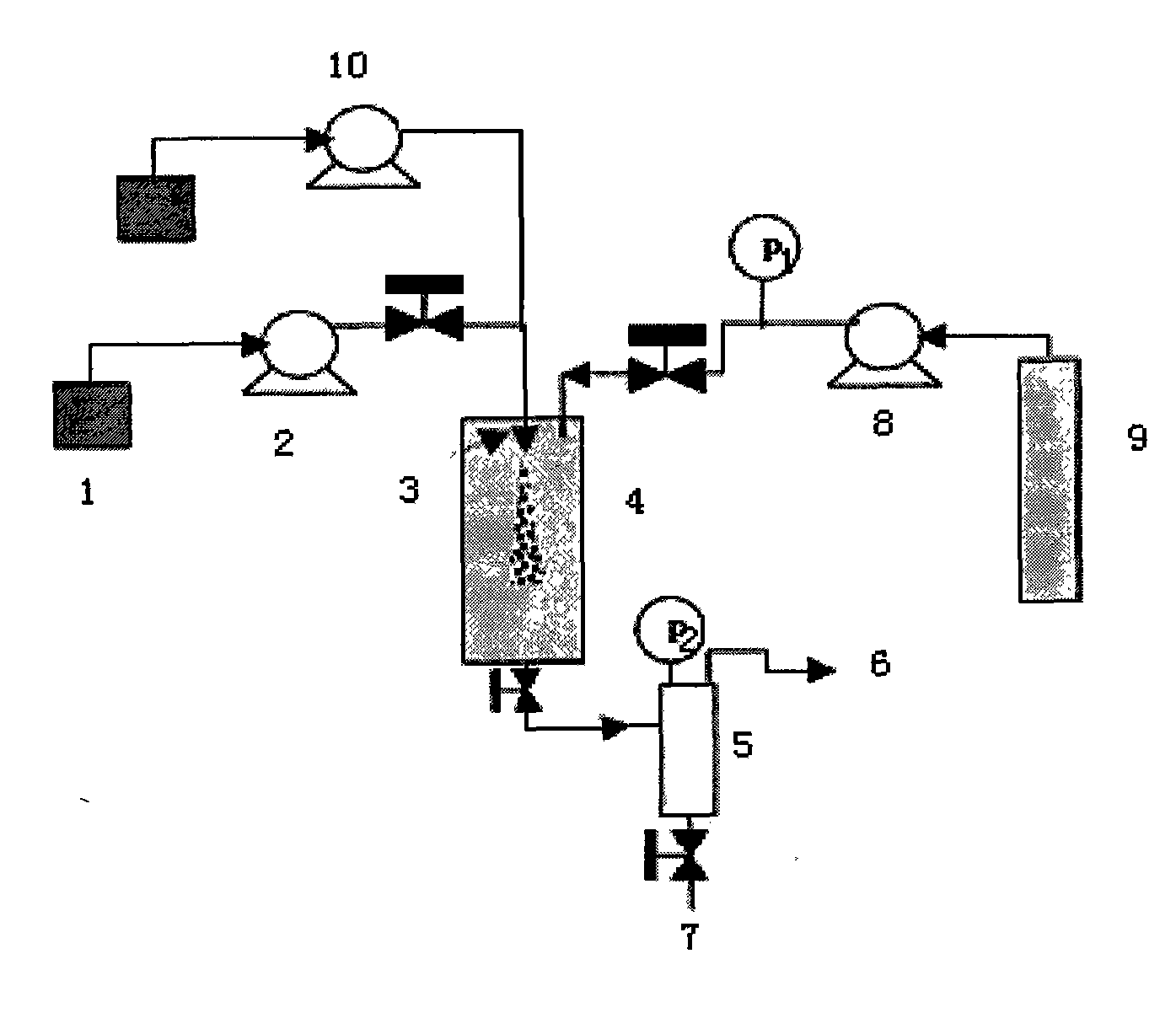
[0027] Example: A preparation process of oridonin fine particles, characterized in that it includes the following steps:
[0028] (1) Configure oridonin solution: fully dissolve oridonin in the organic solvent according to the ratio of organic solvent: oridonin at 100:2, so that the concentration of oridonin is 2.0 %, the dissolution temperature is controlled at 40℃;
[0029] (2) Connect the oridonin solution 1 prepared in step (1) with the solution pump 2, and control the working pressure to 15MPa;
[0030] (3) Carbon dioxide feed: the CO in the cylinder 2 9 Enter the supercritical fluid anti-solvent equipment system through the booster pump valve 8, and enter the crystallization kettle 4, the flow rate is controlled at 10ml / min, the starting temperature is controlled at 45°C; the pressure is 15MPa;
[0031] (4) The oridonin solution configured in the above step (1) is rapidly sprayed into the crystallization kettle 4 through the solution pump 2 through the nozzle 3 in the supercriti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com