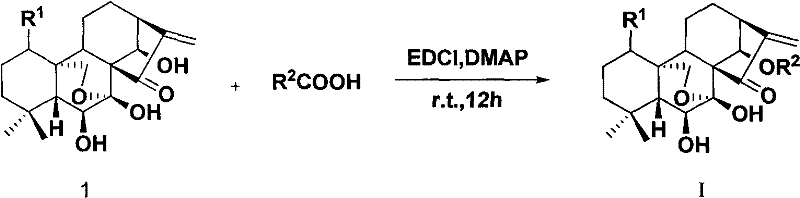Rubescensine A having antitumor activity and fluorine-containing derivatives of 6,7-cyclobebescensine A, preparation method and use
A technology of Rubescensine A and medicinal salts, applied in the fields of natural medicine and medicinal chemistry, and can solve the problems of limited application, poor water solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
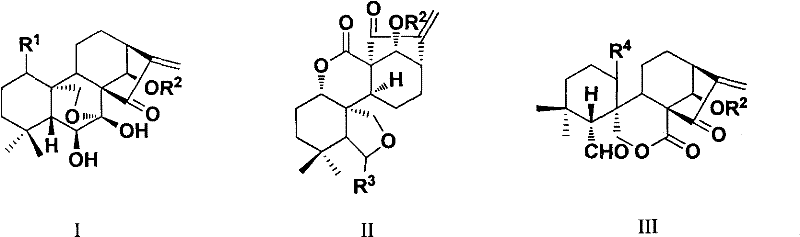
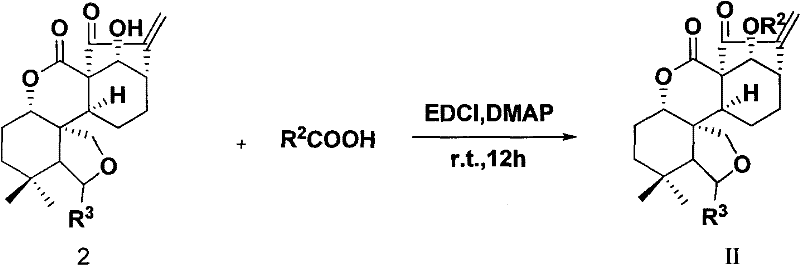
[0122] ent-1α-hydroxy-6β,7β-dihydroxy-[14β-O-(4-trifluoromethylbenzoyl)]-15-oxo-7,20-oxo-16-kaurene
[0123] Dissolve oridonin (72mg, 0.2mmol) in 15ml of dichloromethane, add 4-trifluoromethylbenzoic acid (57mg, 0.3mmol), stir at room temperature for 12 hours, add appropriate amount of water (about 15ml), acetic acid Extracted with ethyl ester (10ml×3 times), washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and column chromatography (dichloromethane:methanol=300:1) gave 58mg of white powdery solid (yield 54% ): m.p.270-275℃; 1 H-NMR (CDCl 3 , 300M Hz), δ (ppm): 1.11 (3H, s, 19-CH 3 ), 1.13 (3H, s, 18-CH 3 ), 1.26 (1H, m, 5-CH), 1.46 (2H, m, 3-CH 2 ), 1.65 (2H, m, 2-CH 2 ), 1.83 (1H, m, 9-CH), 2.02 (2H, m, 11-CH 2 ), 2.33 (1H, m, 12-CH), 2.68 (1H, m, 12-CH 2 ), 3.27 (1H, d, J=9.6Hz, 13-CH), 3.54 (1H, m, 1-CH), 3.76 (1H, m, 6-CH), 3.78 (1H, s, 1-OH) , 4.09, 4.38 (each 1H, dd, J A =J B =10.2Hz, 20-CH 2 ), 5.54 (1H, s, 17-CH 2 )...
Embodiment 2
[0125] ent-1α-hydroxy-6β,7β-dihydroxy-[14β-O-(4-fluorobenzoyl)]-15-oxo-7,20-oxo-16-kaurene
[0126] Reference Example 1: ent-1α-hydroxy-6β, 7β-dihydroxy-[14β-O-(4-trifluoromethylbenzoyl)]-15-oxo-7,20-oxo-16- Synthetic method of kaurene. 58 mg of white solid was obtained (59% yield): m.p.90-93°C; 1 H-NMR (CDCl 3 , 500MHz), δ (ppm): 1.12 (3H, s, 19-CH 3 ), 1.13 (3H, s, 18-CH 3 ), 1.29 (1H, m, 5-CH), 1.48 (2H, m, 3-CH 2 ), 1.64 (2H, m, 2-CH 2 ), 1.80 (1H, m, 9-CH), 2.01 (2H, m, 11-CH 2 ), 2.34 (1H, m, 12-CH 2 ), 2.67 (1H, m, 12-CH 2 ), 3.28 (1H, d, J=9.5Hz, 13-CH), 3.52 (1H, m, 1-CH), 3.76 (1H, m, 6-CH), 3.90 (1H, s, 1-OH) , 4.09, 4.37 (each 1H, dd, J A =J B =10.0Hz, 20-CH 2 ), 5.51 (1H, s, 17-CH 2 ), 6.08 (1H, d, 6-OH), 6.11 (1H, s, 14-CH), 6.19 (1H, s, 17-CH 2 ), 7.06 (2H, m, Ar-H), 7.94 (2H, m, Ar-H). MS (ESI) m / z: 487.2 [M+H] + , 509.2[M+Na] + , 525.2[M+K] + .
Embodiment 3
[0128] ent-1α-hydroxy-6β,7β-dihydroxy-[14β-O-(3-fluorobenzoyl)]-15-oxo-7,20-oxo-16-kaurene
[0129] Reference Example 1: ent-1α-hydroxy-6β, 7β-dihydroxy-[14β-O-(4-trifluoromethylbenzoyl)]-15-oxo-7,20-oxo-16- Synthetic method of kaurene. 60 mg of white solid was obtained (61% yield): m.p.228-230°C; 1 H-NMR (CDCl 3 , 500M Hz), δ (ppm): 1.11 (3H, s, 19-CH 3 ), 1.13 (3H, s, 18-CH 3 ), 1.29 (1H, m, 5-CH), 1.46 (2H, m, 3-CH 2 ), 1.64 (2H, m, 2-CH 2 ), 1.81 (1H, m, 9-CH), 2.02 (1H, m, 11-CH 2 ), 2.33 (1H, m, 12-CH 2 ), 2.68 (1H, m, 12-CH 2 ), 3.28 (1H, d, J=10.0Hz, 13-CH), 3.53 (1H, m, 1-CH), 3.75 (1H, m, 6-CH), 3.87 (1H, s, 1-OH) , 4.10, 4.37 (each 1H, dd, J A =J B =10.0Hz, 20-CH 2 ), 5.53 (1H, s, 17-CH 2 ), 6.09 (1H, d, J=10.0Hz, 6-OH), 6.12 (1H, s, 14-CH), 6.21 (1H, s, 17-CH 2 ), 7.23 (1H, m, Ar-H), 7.37 (1H, m, Ar-H), 7.62 (1H, d, J=10.0Hz, Ar-H), 7.71 (1H, d, J=10.0Hz , Ar-H). MS (ESI) m / z: 487.2 [M+H] + , 509.2[M+Na] + , 525.2[M+K] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com