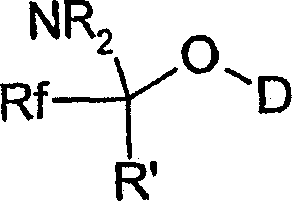
Reagent and method for preparing a fluorinated and silylated derivative
A technology of silylation and derivatives, which is applied in the field of synthesis of silylation derivatives, and can solve problems such as impossibility of industrial application, excessive cost, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0112] Embodiment 1: Utilize various alkalis to carry out qualitative test
[0113] A series of experiments were carried out using various bases.
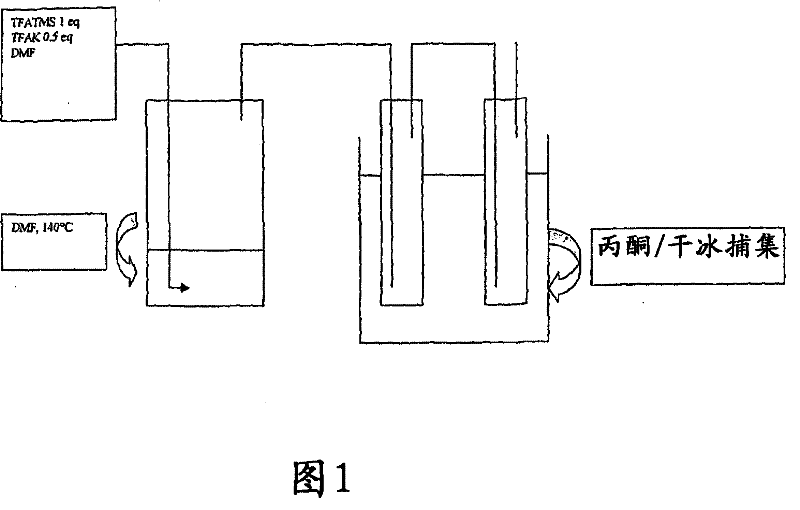
[0114] The base (1 mmol) was charged into a 60 ml Schott tube under argon atmosphere. DMF (2ml) was added at 20°C under an argon atmosphere, followed by trimethylsilyl trifluoroacetate (372mg, 2mmol).
[0115] The tube was closed and the reaction mixture was heated at 140 °C for the desired time.
[0116] After returning to 20° C., the reaction medium was analyzed without further processing.
[0117] The properties are summarized in the table below.
[0118] Table (I)
[0119]
[0120] *:ordinary
[0121] **:medium
[0122] ***:good
[0123] ****:very good
[0124] *****: Excellent
Embodiment II
[0125] Example II: Trimethylsilyl trifluoroacetate in the presence of potassium trifluoroacetate
[0126] Potassium trifluoroacetate (152 mg, 1 mmol) was charged to a 60 ml Schott tube under argon atmosphere.
[0127] DMF (2ml) was added at 20°C under an argon atmosphere, followed by trimethylsilyl trifluoroacetate (372mg, 2mmol).
[0128] The tube was closed and the reaction mixture was heated at 140 °C for the desired time.
[0129] After returning to 20° C., the reaction medium was analyzed without further processing.
[0130] The properties are summarized in the table below.
[0131] Table (II)
[0132] test
[0133] (a) by using the internal standard 19 F NMR measurement (b) RY=8% relative to TMSTFA, RY=75% relative to KTFA.
Embodiment III
[0134] Example III: Trimethylsilyl trifluoroacetate in the presence of potassium chloride
[0135] Potassium chloride (74.5 mg, 1 mmol) was charged to a 60 ml Schott tube under argon atmosphere.
[0136] DMF (2ml) was added at 20°C under an argon atmosphere, followed by trimethylsilyl trifluoroacetate (372mg, 2mmol).
[0137] The tube was closed and the reaction mixture was heated at 140 °C for the desired time.
[0138] After returning to 20° C., the reaction medium was analyzed without further processing.
[0139] The properties are summarized in the table below.
[0140] Table (III)
[0141] test
[0142] (a) by using the internal standard 19 F NMR determination (b) source of chloride: Me 4 NCl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com