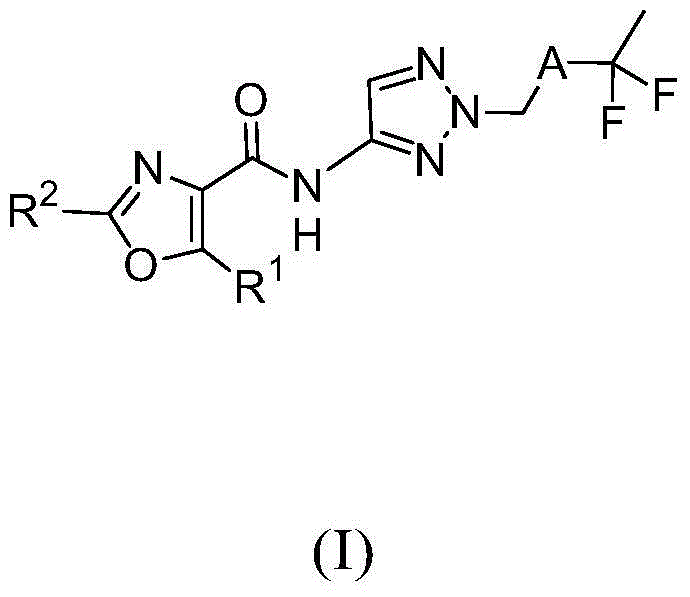
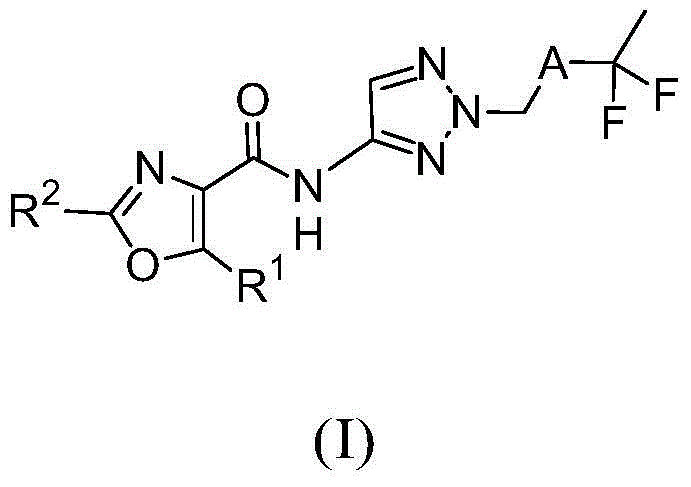
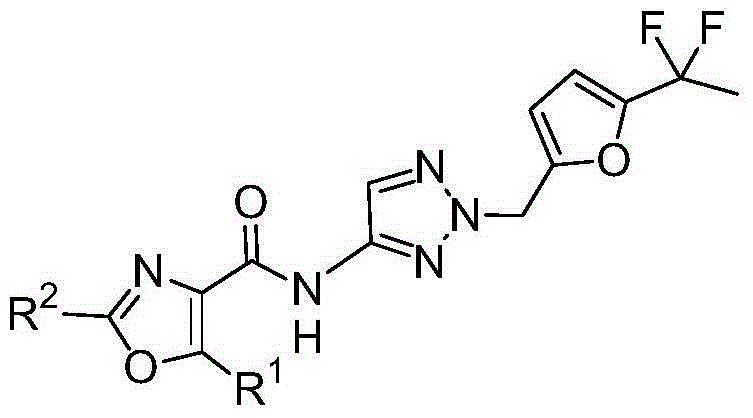
Fluorinated aminotriazole derivatives
A compound, trifluoromethyl technology, applied in the field of ALX receptor activator, can solve neuron toxicity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0576] 2-Methyl-5-m-tolyl-oxazole-4-carboxylic acid {2-[5-(1,1-difluoro-ethyl)-furan-2-ylmethyl]-2H-[1 ,2,3]triazol-4-yl}-amide:
[0577] Following general procedure A, from 2-[5-(1,1-difluoro-ethyl)-furan-2-ylmethyl]-2H-[1,2,3]triazol-4-ylamine with 2 -Methyl-5-m-tolyl-oxazole-4-carboxylic acid begins.
[0578] LC-MS-Conditions 02:t R =1.12 minutes; [M+H] + =427.83.
Embodiment 2
[0580]5-Phenyl-oxazole-4-carboxylic acid {2-[4-(1,1-difluoro-ethyl)-thiazol-2-ylmethyl]-2H-[1,2,3]triazole -4-yl}-amide:
[0581] Following general procedure A, from 2-[4-(1,1-difluoro-ethyl)-thiazol-2-ylmethyl]-2H-[1,2,3]triazol-4-ylamine with 5 -Phenyl-oxazole-4-carboxylic acid to start.
[0582] LC-MS-Conditions 01:t R =1.01 minutes; [M+H] + =416.90.
Embodiment 3
[0584] 2-Methyl-5-m-tolyl-oxazole-4-carboxylic acid {2-[4-(1,1-difluoro-ethyl)-thiazol-2-ylmethyl]-2H-[1 ,2,3]triazol-4-yl}-amide:
[0585] Following general procedure A, from 2-[4-(1,1-difluoro-ethyl)-thiazol-2-ylmethyl]-2H-[1,2,3]triazol-4-ylamine with 2 -Methyl-5-m-tolyl-oxazole-4-carboxylic acid begins.
[0586] LC-MS-Conditions 02:t R =1.10 minutes; [M+H] + =445.09.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com