Water soluble oridonin derivative and preparation method thereof
A water-soluble derivative technology, applied in the field of water-soluble oridonin derivatives and its preparation, can solve the problems of bitter taste, poor water solubility, limited application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
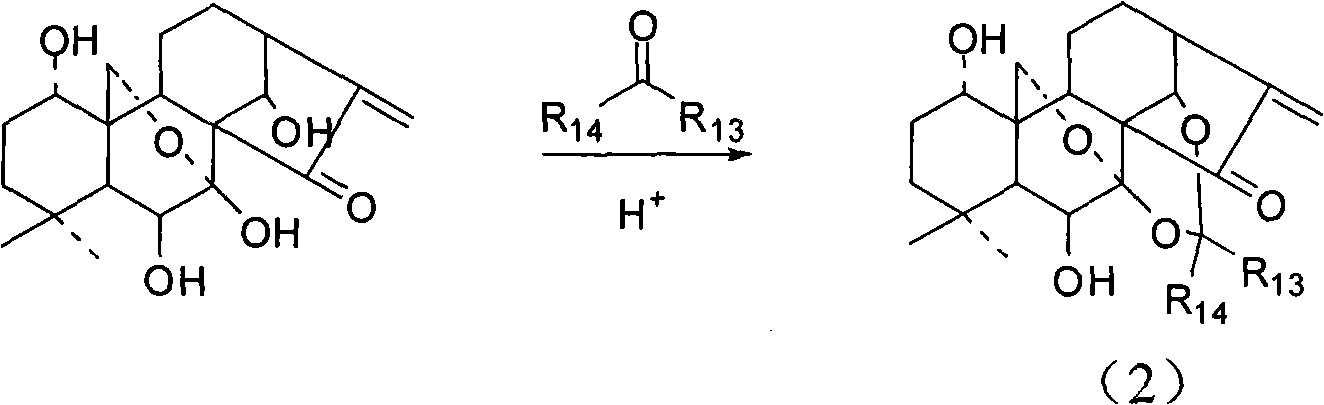
[0043] The synthesis of the oridonine A of embodiment 1 propylidene protection
[0044] Under nitrogen protection, 10 mg p-toluenesulfonic acid was added to 0.3 g (0.823 mmol) oridonin, 0.51 ml 2,2-dimethoxypropane (4.12 mmol) and acetone mixture, stirred at room temperature for 6 Hour. Add sodium bicarbonate powder to neutralize and evaporate to dryness. The residue was purified with neutral alumina and eluted with PE:EA=1:1 to obtain 3.27 g of propylidene-protected orididenin (yield=98%) as a pure white solid powder. H1 NMR (CDCl3): 6.14(s, 1H), 5.73(d, J=11.2Hz, 1H), 5.54(s, 1H), 4.78(s, 1H), 4.23(d, J=10Hz, 1H), 4.05(d, J=10.0Hz, 1H), 3.91(m, 1H), 3.46(m, 1H), 3.05(d, J=8.8Hz, 1H), 2.53(m, 1H), 1.95(m, 1H ), 1.81-1.43 (m, 10H), 1.73 (s, 3H), 1.33 (s, 3H), 1.17 (s, 3H), 1.15 (s, 3H).
Embodiment 2
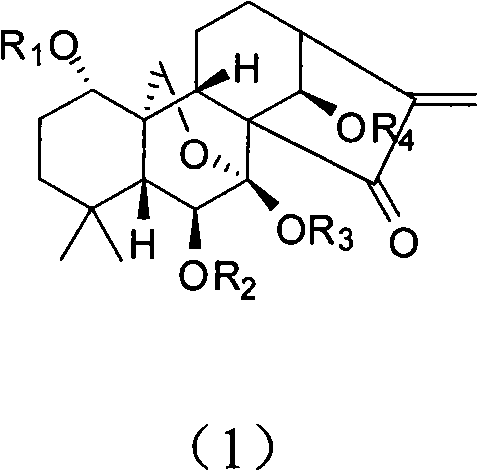
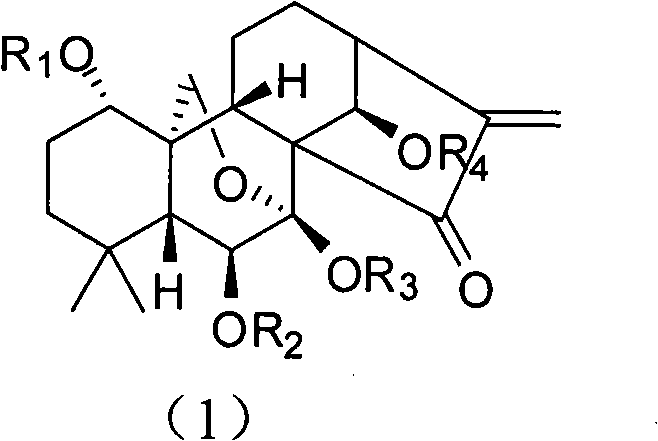
[0046] When R1 represents CO(CH 2 ) 2 CO 2When Na, R2, R3 and R4 represent H, the preparation of Oridonin A derivative shown in general formula 1: under nitrogen protection, 74mg succinic anhydride (0.74mmol) is added to 100mg propylidene protected Oridonin In a mixture of methyl element (0.247mmol) and pyridine, heated to 80°C, stirred for 24 hours, and evaporated to dryness. The residue was applied to a macroporous adsorption resin column AB-8, washed with water with a pH value of 4, then with water with a pH value of 7.0, and finally eluted with ethanol, and concentrated to obtain a white solid. The white solid was dissolved in methanol and strongly acidic cationic resin and stirred for 2 days, filtered, the filtrate was concentrated, purified by reverse phase column, eluted with methanol / water, and neutralized with NaOH to obtain Oridonin A Sodium Succinate. H1 NMR (D2O): 6.16(s, 1H), 5.68(s, 1H), 4.96(s, 1H), 4.33(d, J=10.4Hz, 1H), 3.70(d, J=6.0Hz, 1H) , 3.10(d, J=10....
Embodiment 3
[0048] When R1 represents PO 3 Na 2 , when R2, R3 and R4 represent H, the preparation of Oridonin A derivative shown in general formula 1: under nitrogen protection, 13 μl phosphorus oxychloride adds (0.15mmol) to 20mg propylidene protected Oridonin In a mixture of A (0.05 mmol) and pyridine, stirred at room temperature for 24 hours, and evaporated to dryness. The residue was applied to a macroporous adsorption resin column AB-8, washed first with water with a pH value of 4, then with water with a pH value of 7.0, and finally eluted with ethanol, and concentrated to obtain 15 mg of a white solid. The white solid was dissolved in methanol and strong acidic cationic resin and stirred for 2 days, filtered, the filtrate was concentrated, purified by reverse phase column, eluted with methanol / water, and neutralized with NaOH to obtain oridonin sodium phosphate. H1 NMR (D2O): 6.24(s, 1H), 5.77(s, 1H), 5.10(s, 1H), 4.36(d, J=10.4Hz, 1H), 4.20(d, J=10.4Hz, 1H) , 3.94(m, 1H), 3.79(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com