L-alanine-(14-oridonin) ester trifluoroacetate as well as preparation method and application thereof
A technology of ester trifluoroacetate and oridonin A, applied in the field of L-alanine-ester trifluoroacetate and its preparation, can solve the difficulty of intravenous administration, reduce activity, and fail to reach blood drug Concentration and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
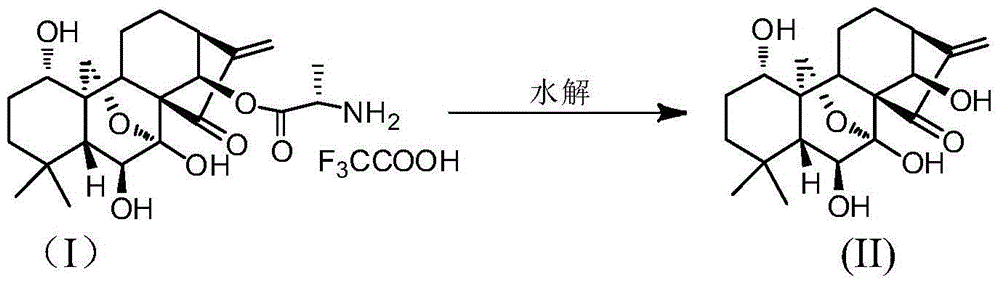
[0027] Preparation of N-Boc-L-alanine-(14-Oridonin A) Ester (III)
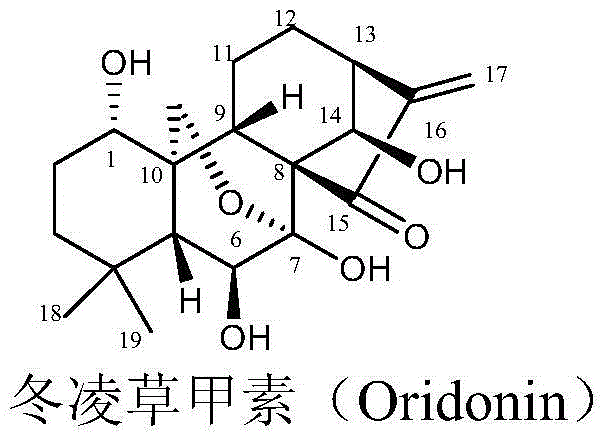
[0028] 150g (0.41mol) oridonin (II), 195g (1.04mol) BOC-L-Ala was suspended in 1.35kg dichloromethane, cooled to 0-5°C in an ice bath, and 213g (1.04mol) bicyclic Hexylcarbodiimide (DCC), stirred for 0.5h, removed the ice bath, stirred at room temperature for 5h, TLC confirmed the complete reaction of raw materials (dichloromethane:methanol=10:1, raw material R f =0.4, product R f =0.6). The reaction solution was cooled to 0°C and stood for 2h, filtered, and the filter cake was washed with dichloromethane (300g×3). The organic layers were combined and concentrated to obtain a white solid. Flash silica gel column chromatography (dichloromethane:methanol=100:1~60:1; v / v), collect product components, concentrate to dryness under reduced pressure to obtain about 140~165g of white solid powder. Add 600g of isopropyl ether to the above white powder, stir and wash for 2 hours, filter, wash the filter cake with iso...
Embodiment 2
[0031] Preparation of L-alanine-(14-oridonin A) ester trifluoroacetate (I)
[0032] 120g (0.224mol) of N-BOC-L-alanine-(14-oridonin A) ester (III) was dissolved in 600g of dichloromethane and cooled to 0°C. Slowly add 2.5kg of trifluoroacetic acid / dichloromethane (1:1; w / w) mixed solution dropwise at 0-5°C, and the addition is completed within 20 minutes. Stir at 0-5°C for 30 minutes, TLC confirmed that the reaction was complete (dichloromethane:methanol=10:1, raw material R f =0.6, product R f =0.3), the reaction solution was concentrated under reduced pressure to obtain light yellow to light red oil. After adding 7.2kg of isopropyl ether and stirring, a large amount of light yellow solid was precipitated. After stirring for 1 hour, it was filtered, and the filter cake was washed with isopropyl ether (145g×3). Dry in a vacuum oven to obtain 105-116 g of off-white or light yellow solid, with a yield of 85-94%. (HPLC>98%).
[0033]1 H-NMR (400MHz,MeOD):6.16(s,1H),6.10(s,1H...
Embodiment 3
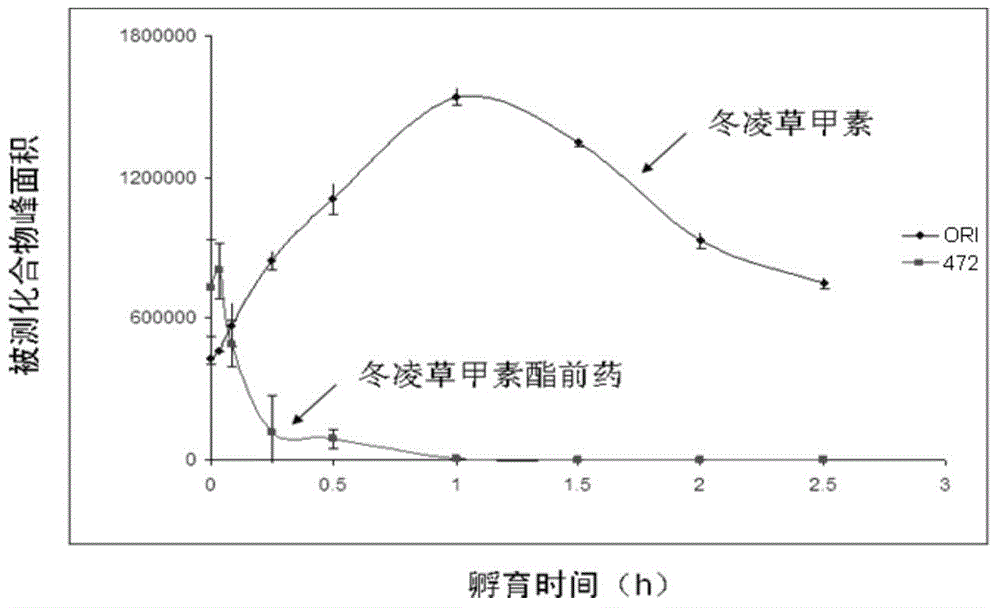
[0036] According to the synthetic method of embodiment 1,2, the obtained L-alanine-(14-oridonin) ester trifluoroacetate, the acceleration of three batches of samples (25 ℃ ± 2 ℃, RH60% ± 10 %), long-term (2 ~ 8 ℃, RH60% ± 10%) stability investigation is shown in Table 1 and Table 2 below, the experimental data results show that the stability of the sample is good.
[0037] Table 1. Accelerated (25°C±2°C, RH60%±10%) test results
[0038]
[0039] Note: "--" means not detected.
[0040] Table 2. Long-term (2-8°C, RH60%±10%) test results
[0041]
[0042] Note: "--" means not detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com