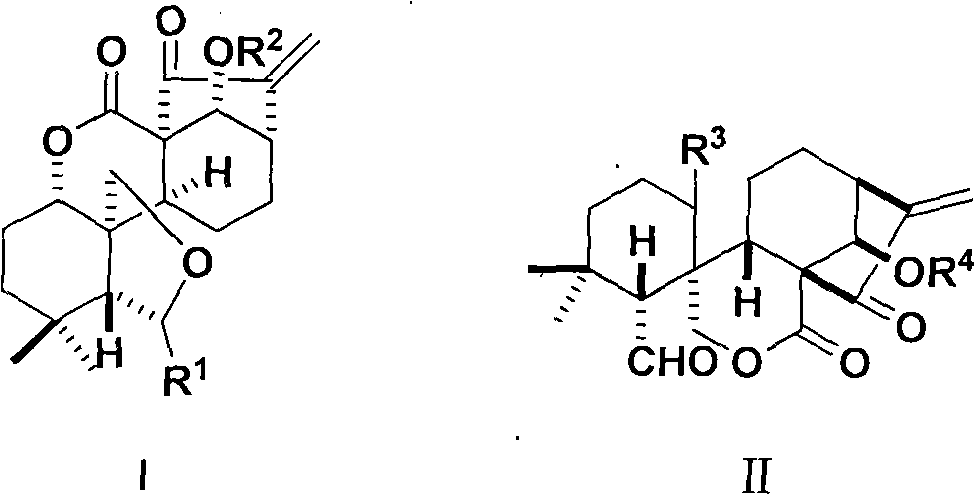
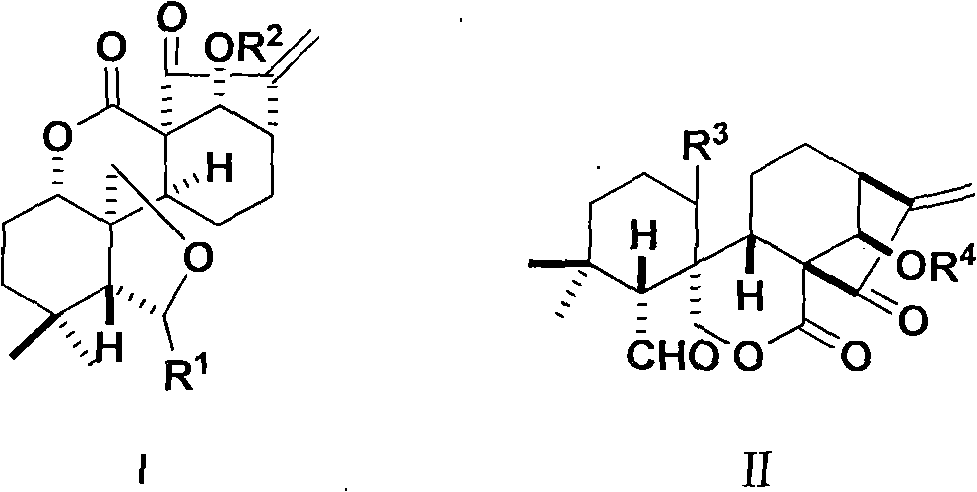
Ent-6,7-open-cycle kaurene type rubescensine a derivative with Anti-tumor activity and preparation method and use thereof
A technology of ent-6 and kaurene, which is applied in the fields of natural medicine and medicinal chemistry, and can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] ent-6,14-dihydroxy-7,15-dioxo-1,7-lactone-6,20-hemiketal-6,7-cyclo-16-kaurene
[0108] Oridonin (72mg, 0.2mmol) was dissolved in 10ml of water, sodium periodate (644g, 3mmol) was added, stirred at room temperature for 24 hours, extracted three times with dichloromethane, each 10ml, washed twice with saturated saline, without Dry over sodium sulfate, filter, concentrate (the crude product can be directly used in the next reaction), and column chromatography (dichloromethane:methanol=80:1) gives a white powdery solid (65 mg, 91.4%). 1 H-NMR (DMSO, 500MHz), δ (ppm): 6.17 (1H, d, J = 3.3Hz, 6-OH), 5.92 (1H, s, 17-CH 2 ), 5.52 (1H, s, 17-CH 2), 5.38 (1H, d, J = 2.1Hz, 14-OH), 5.17 (1H, d, J = 2.1Hz, 6-CH), 4.67 (1H, s, 14-CH), 4.65 (1H, m , 1-CH), 3.87, 3.58 (each1H, dd, J A =J B =9.1Hz, 20-CH 2 ), 2.95 (1H, d, J=9.1Hz, 13-CH), 2.49 (2H, m, 12-CH 2 ), 1.67 (2H, m, 2-CH 2 ), 1.36 (2H, m, 11-CH 2 ), 1.26 (1H, m, 9-CH), 0.93 (3H, s, 18-CH 3 ), 0.88 (3H, s, 19-CH 3 ). ...
Embodiment 2
[0110] ent-6,7,15-trioxo-1,7-lactone-6,20-lactone-14-hydroxy-6,7-cyclo-16-kaurene
[0111] Ent-6,14-dihydroxy-7,15-dioxo-1,7-lactone-6,20-hemiketal-6,7-cyclo-16-kaurene (72mg, 0.2mmol ) was dissolved in 10ml of acetone, added 0.5ml of Jones’ reagent and stirred at room temperature for 10min, added 0.3ml of isopropanol, evaporated the acetone under reduced pressure, extracted three times with 10ml of dichloromethane, washed twice with saturated saline, dried over anhydrous sodium sulfate , filtered, concentrated (the crude product can be directly used in the next reaction), and column chromatography (dichloromethane:methanol=100:1) gave a white powdery solid (63mg, 87.8%). 1 H-NMR (CDCl 3 , 300MHz), δ (ppm): 6.41 (1H, s, 17-CH 2 ), 5.69 (1H, s, 17-CH 2 ), 4.55 (1H, s, 14-CH), 4.45 (1H, m, 1-CH), 4.35, 4.01 (each1H, dd, J A =J B =10.2Hz, 20-CH 2 ), 3.19 (1H, d, J=9.3Hz, 13-CH), 1.21 (3H, s, 18-CH 3 ), 1.07 (3H, s, 19-CH 3 ). MS-ESI(m / z): 719.1[2M-H] - , 359.1[M-H] - ,...
Embodiment 3
[0113] ent-6-hydroxy-7,15-dioxo-1,7-lactone-6,20-hemiketal-(14-O-pentanoyl)-6,7-cyclo-16-kaurene
[0114] Ent-6,14-dihydroxy-7,15-dioxo-1,7-lactone-6,20-hemiketal-6,7-cyclo-16-kaurene (72mg, 0.2mmol ) was dissolved in 10 ml of anhydrous dichloromethane, and 0.5 ml of triethylamine was added dropwise under ice-cooling. After stirring for 10 minutes, 0.5 ml of valeryl chloride was added dropwise and reacted for 15 minutes. Washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and column chromatography (dichloromethane:methanol=300:1) gave a pale yellow solid (73mg, 83.3%). 1 H-NMR (CDCl 3 , 300MHz), δ (ppm): 6.27 (1H, s, 17-CH 2 ), 6.15 (1H, s, 6-CH), 5.63 (1H, s, 17-CH 2 ), 4.56 (1H, s, 14-CH), 4.44 (1H, m, 1-CH), 4.41, 4.031 (each1H, dd, J A =J B =9.3Hz, 20-CH 2 ), 3.19 (1H, d, J=8.1Hz, 13-CH), 1.25 (3H, s, 18-CH 3 ), 1.02 (3H, s, 19-CH 3 ), 0.88 (3H, s, -CH 3 ). MS-ESI(m / z): 464.1[M+NH4] + , 447.1[M+H] + , 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com