Oridonin derivative and preparation method thereof
A technology of Rubescensin A and Rubescensin A, applied in drug combination, organic chemistry, anti-tumor drugs, etc., can solve the problems of poor water solubility, limited use, and insufficient anti-tumor activity of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
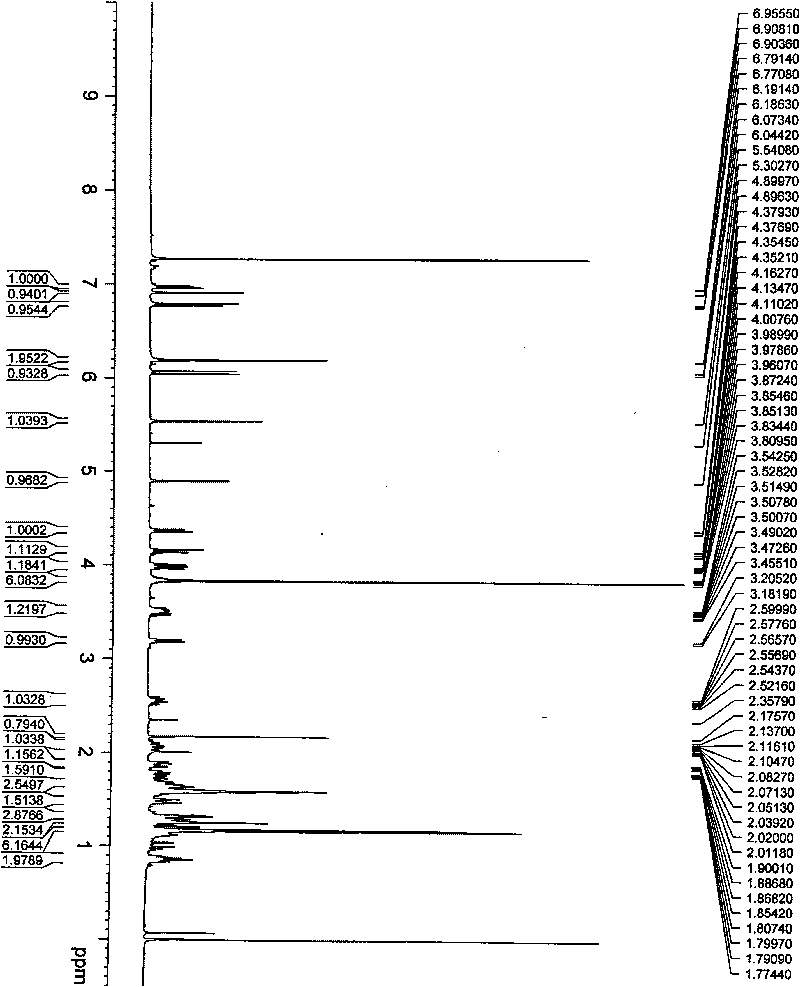
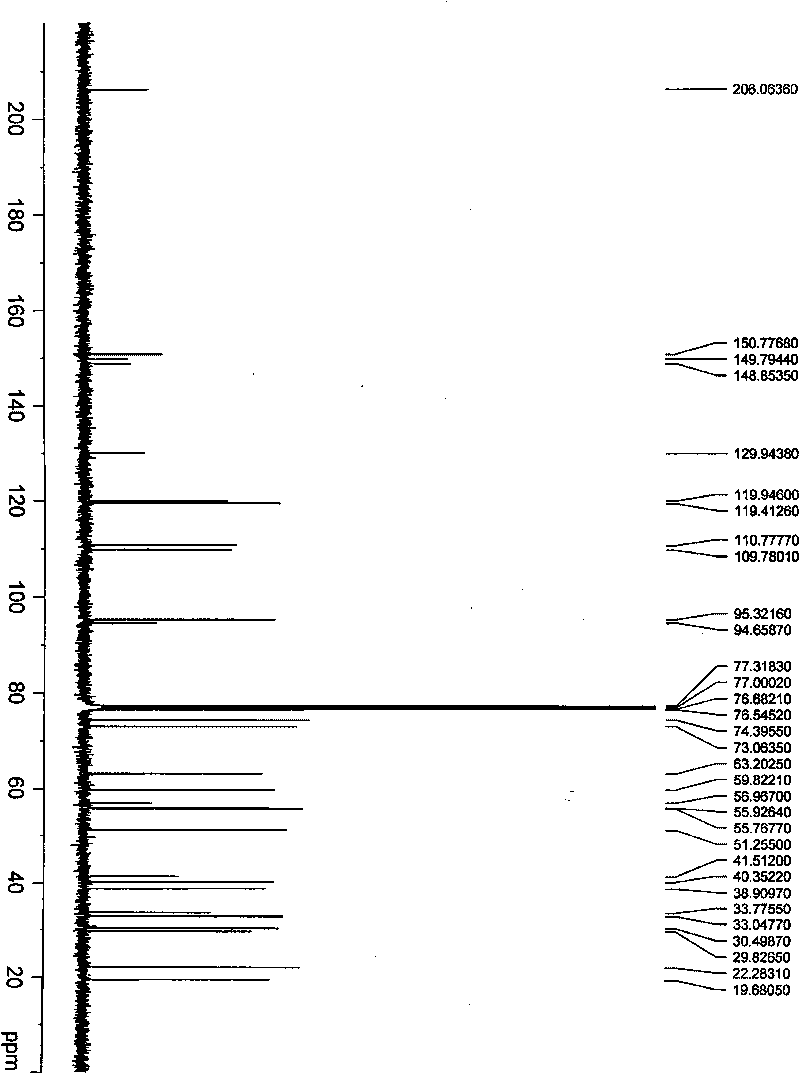
[0026] Example 1 when R 1 =R 2 = 3', 4'-dimethoxybenzylidene, the oridonin derivative (7,14-(3',4'-dimethoxy)benzylidene shown in general formula 1 Forbescensine A, the preparation of compound II):
[0027]
[0028] In a 250ml round bottom flask, add 5g oridonin and 100ml toluene, add 8g 3,4-dimethoxybenzaldehyde and 0.2g p-toluenesulfonic acid under stirring, and react at 80°C for 4 hours. Stop the reaction, extract with water and ethyl acetate, wash the organic phase with saturated sodium bicarbonate solution and saturated ammonium chloride solution, add anhydrous sodium sulfate to dry overnight, filter, and the filtrate is concentrated under reduced pressure to obtain a white solid, which is subjected to silica gel column chromatography , and eluted with petroleum ether-acetone (6:1) to obtain 6.5 g of colorless needle-like crystals with a yield of 92%. M.p.252~254°C.
[0029] Compound Molecular Formula: C 29 h 36 o 8
[0030] The spectrogram data is as follows: ...
Embodiment 2
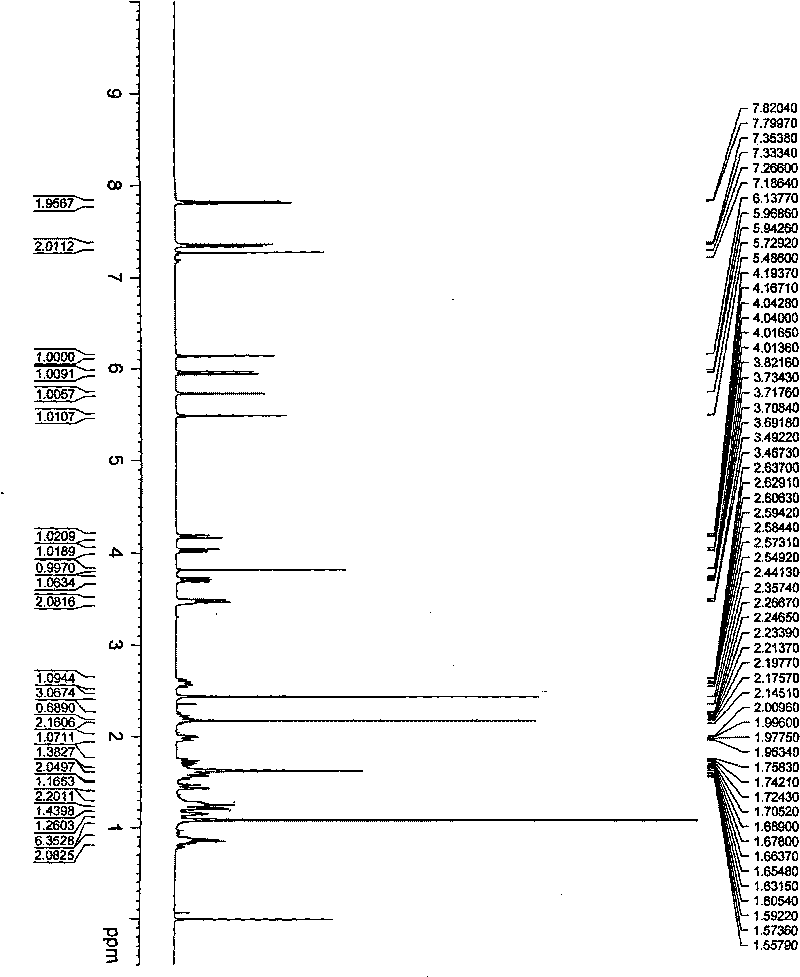
[0035] Example 2 when R 1 = p-toluenesulfonyl, R 2 When =H, the preparation of Rubescensine A derivative (14-tosyl oridonine A, compound III) shown in general formula 1:
[0036]
[0037] In a 250ml round bottom flask, add 5g oridonin, 150ml dichloromethane and 2ml triethylamine, add 5g p-toluenesulfonyl chloride under stirring, after reacting at 0°C for 4 hours, add a small amount of water to quench the reaction, water and Extract with ethyl acetate, wash the organic phase with saturated sodium bicarbonate solution and saturated ammonium chloride solution, add anhydrous sodium sulfate to dry overnight, filter, and concentrate the filtrate under reduced pressure to obtain a white solid, which is recrystallized from petroleum ether-acetone to obtain a colorless 6.8 g of needle-like crystals, yield 95%. M.p.260~262℃.
[0038] Compound Molecular Formula: C 27 h 34 o 8 S
[0039] The spectrogram data is as follows: IR v max KBr (cm -1 ): 3557, 3377, 2951, 2868, 1713, ...
Embodiment 3
[0043] Example 3 When R 1 =R 2 =3',4'-dimethoxybenzylidene, the Rubescensin A derivative represented by the general formula 2 (1-carbonyl 7,14-(3',4'-dimethoxy) ) Preparation of Rubescensine A, Compound IV):
[0044] The first step (preparation of 1-carbonyl Rubescensin A): in a 100ml round bottom flask, add 0.5g Rubescensin A, 40ml acetone, slowly drop 6ml Jone's reagent under stirring at 0°C, and react at room temperature for 2 hours Then, add 0.4 ml of isopropanol, react for 0.5 hour, add 0.5 g of sodium bicarbonate, react for 0.5 hour, add 50 ml of ethyl acetate to dilute, filter, and concentrate the filtrate under reduced pressure to obtain a white solid, which is recrystallized with petroleum ether-acetone to obtain Colorless needle crystals 0.423 g, yield 85%. M.p.220~222℃. ESI-MS(m / z): 385.4([M+Na] + ); 1 H-NMR (400MHz, Acetone) δ: 6.53 (1H, s, 14-OH), 6.10 (1H, s, 17-H), 5.62 (1H, s, 17-H), 5.40 (1H, d, J = 11.3 Hz, 6-OH), 5.24 (1H, s, 7-OH), 4.90 (1H, s, 14-H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com