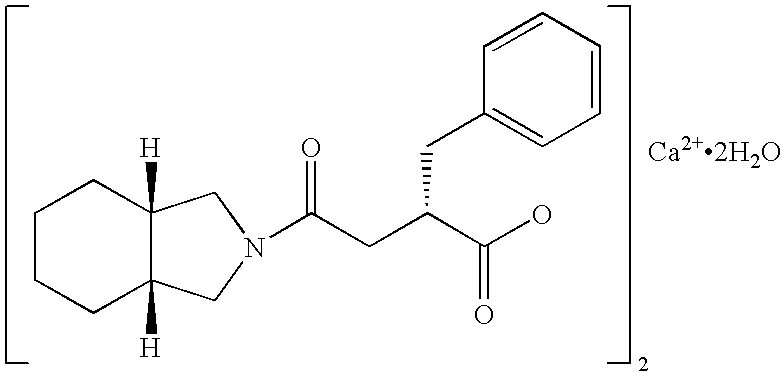
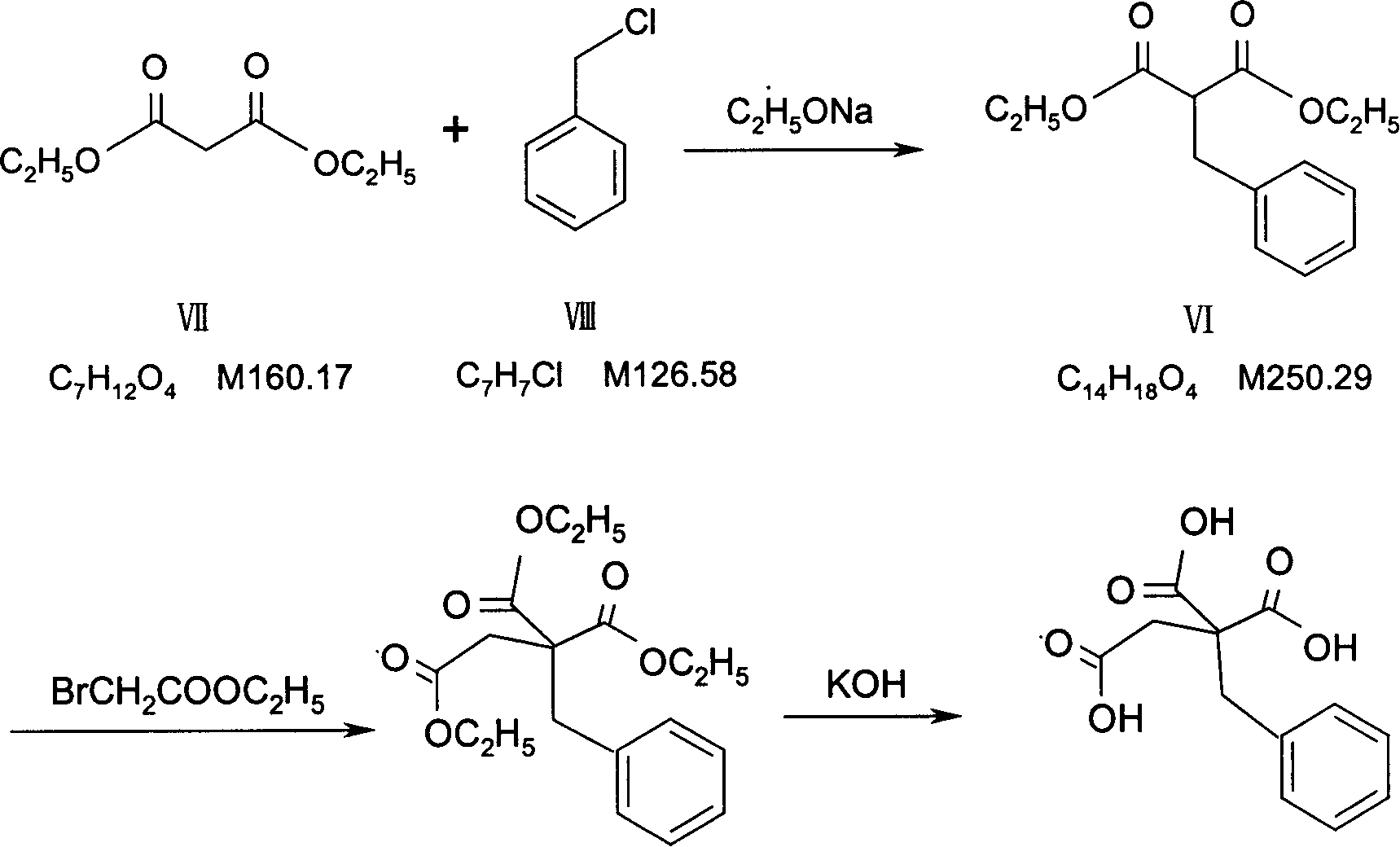
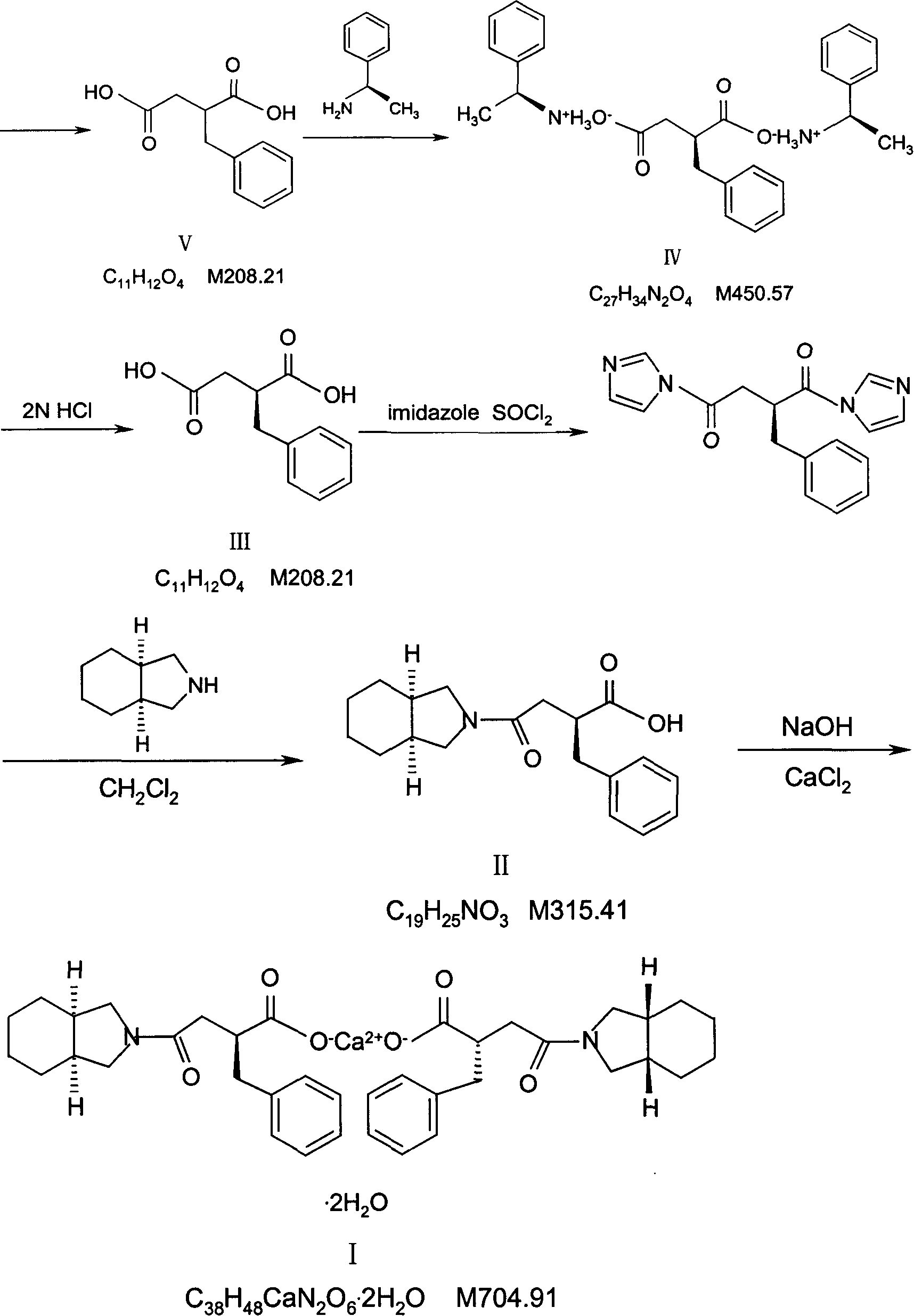
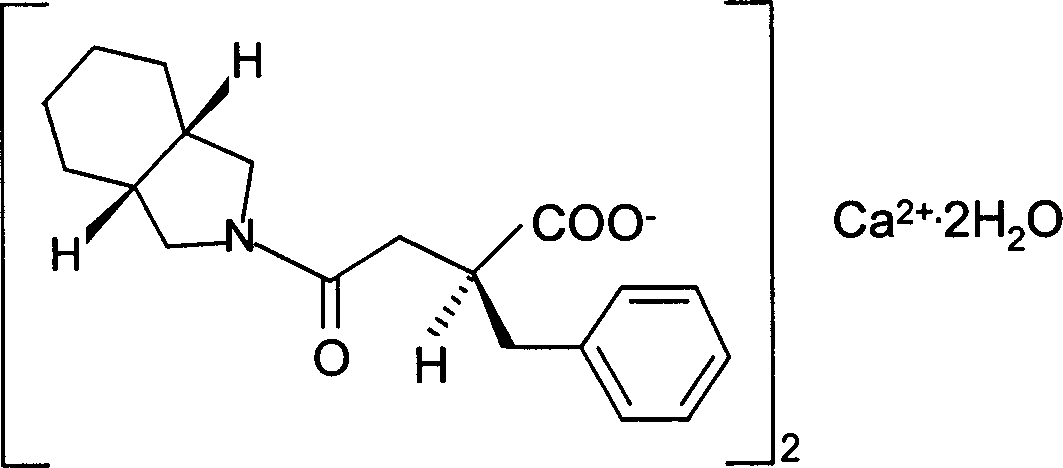
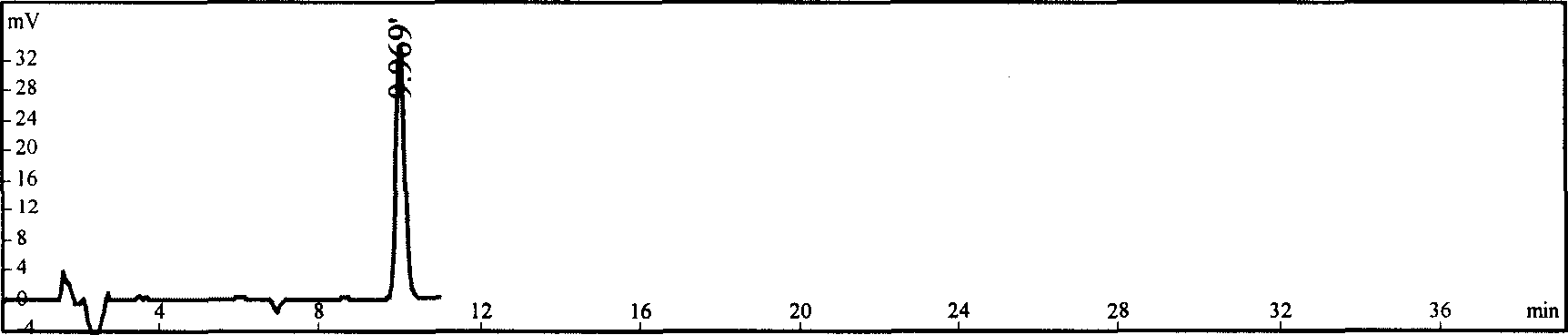
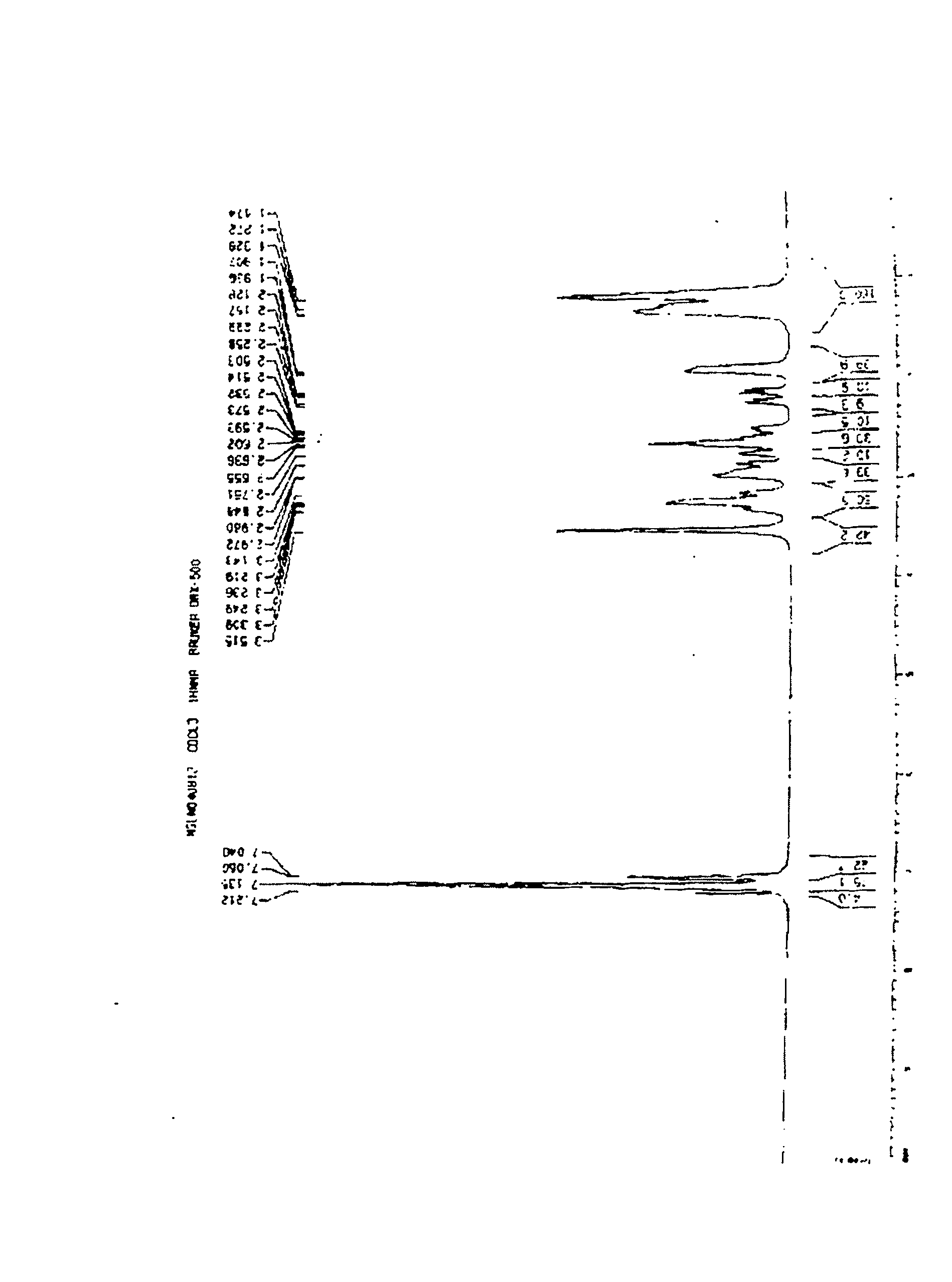
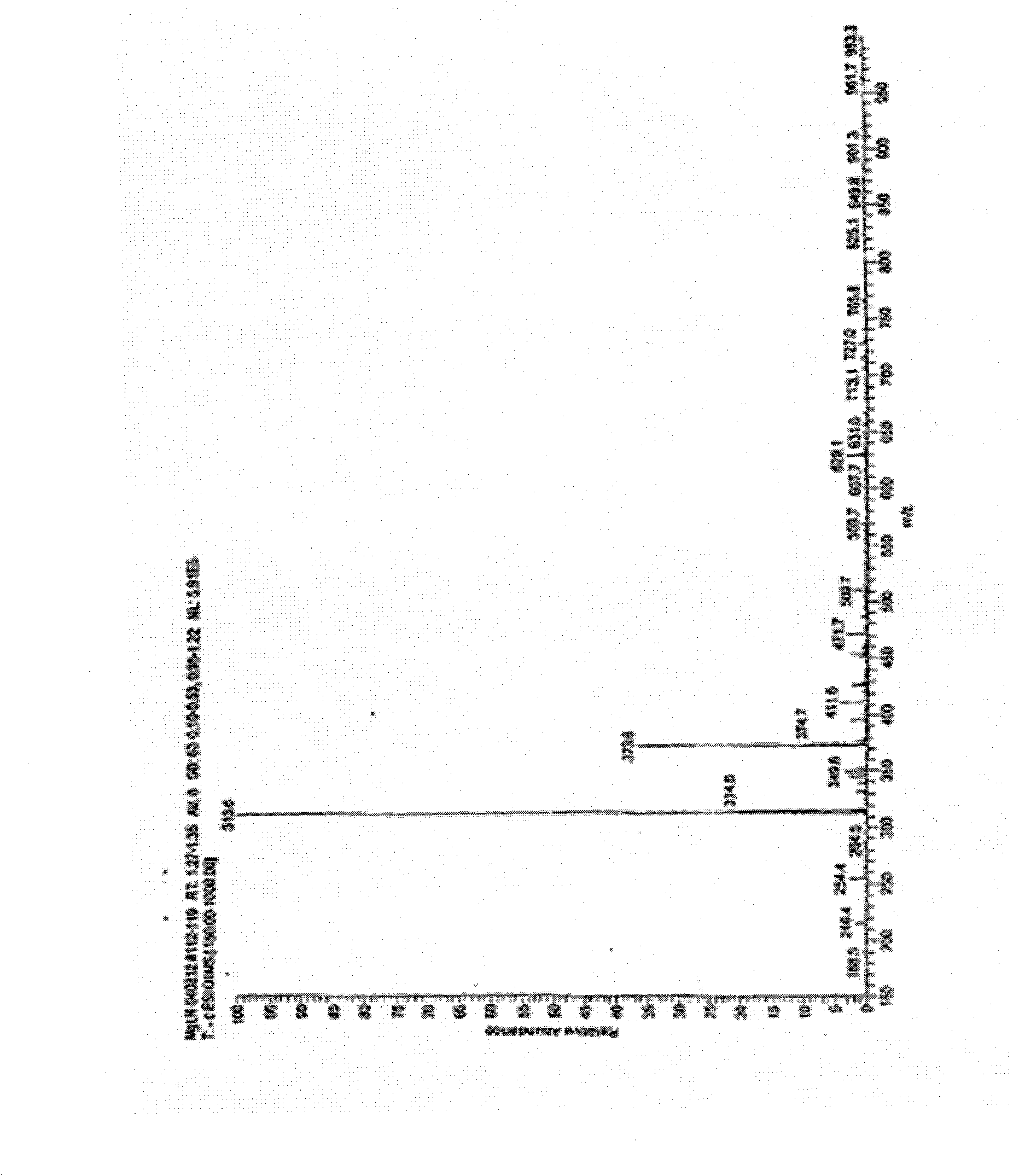
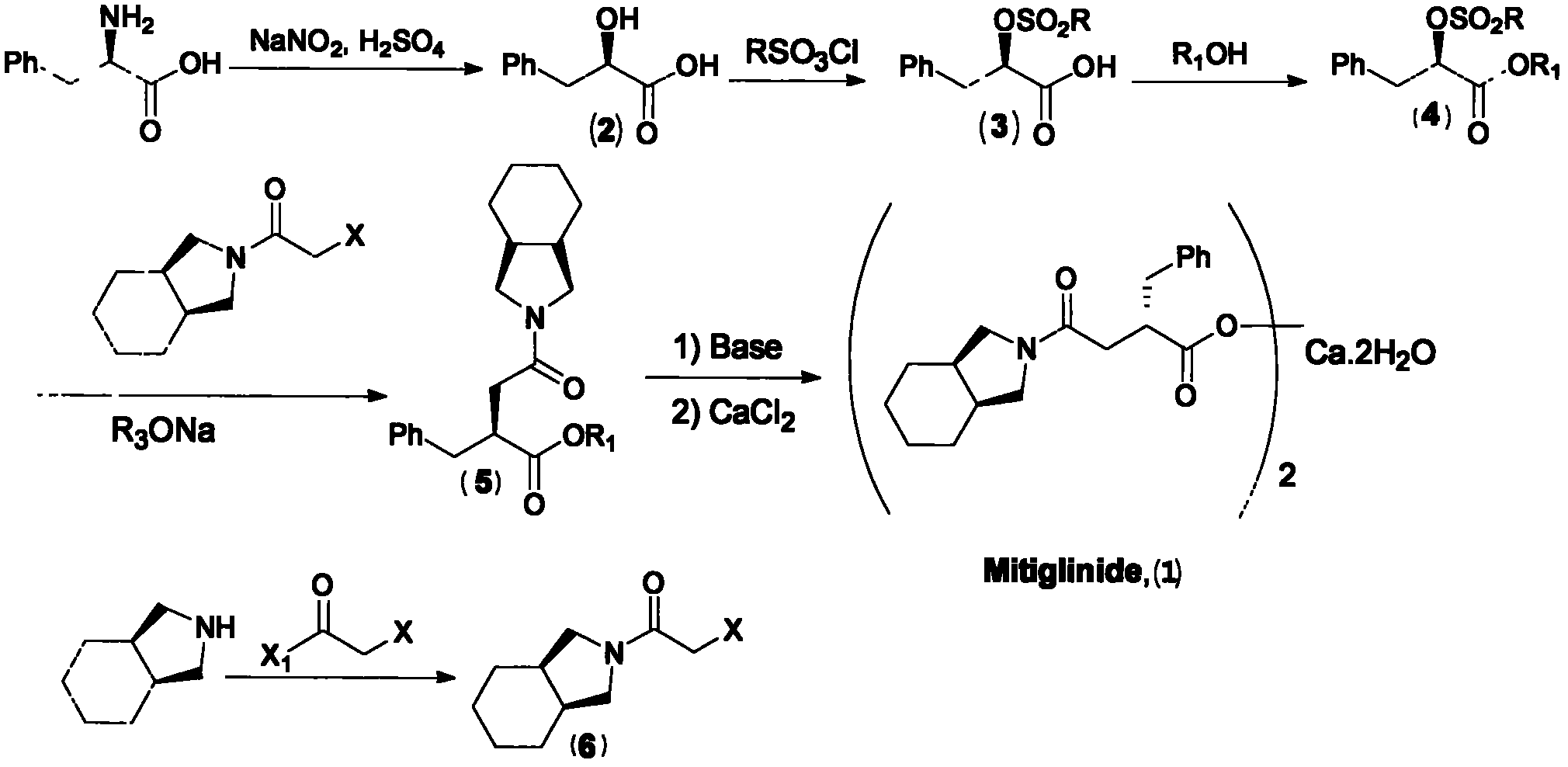
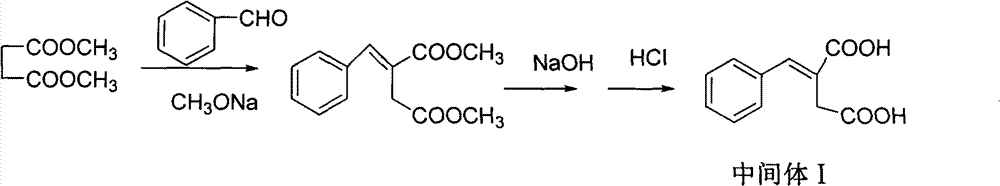
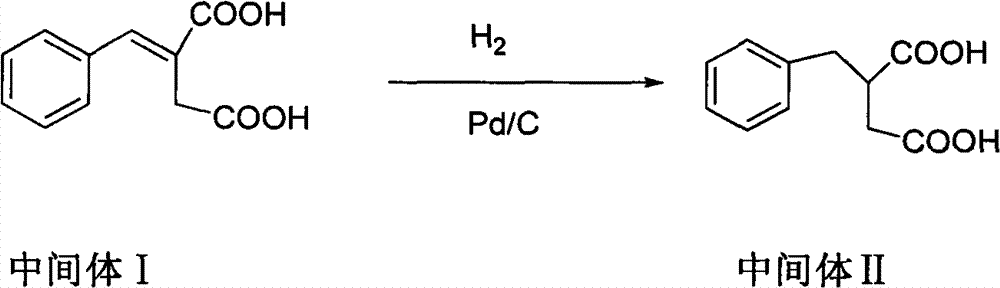
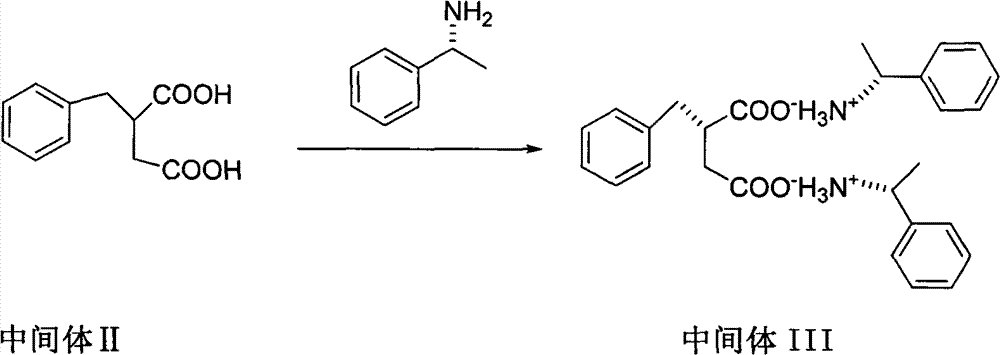
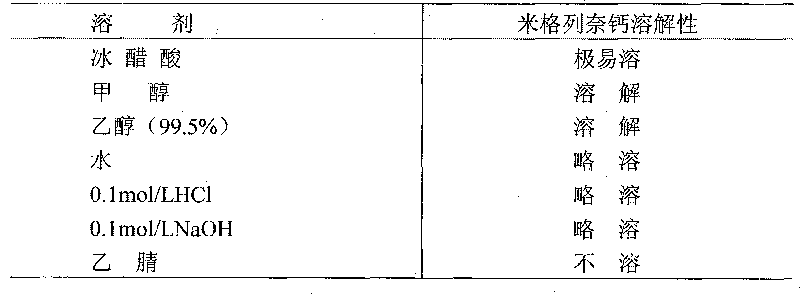
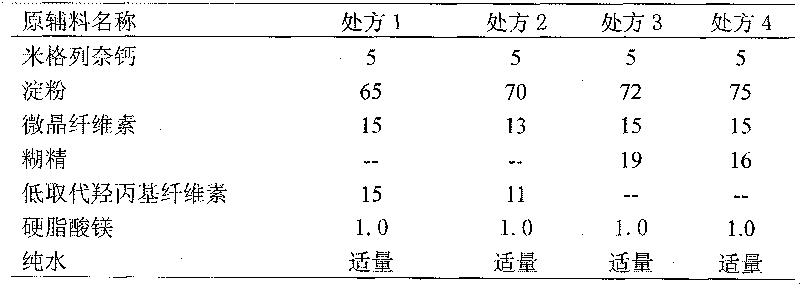
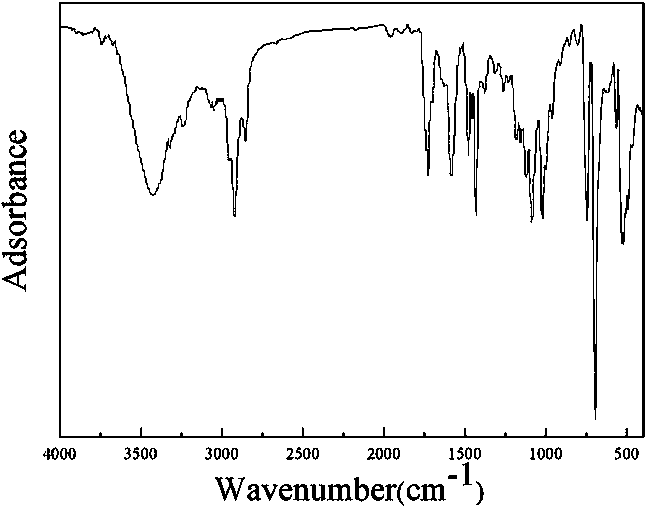
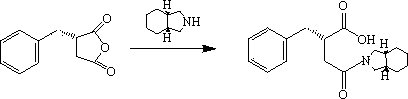
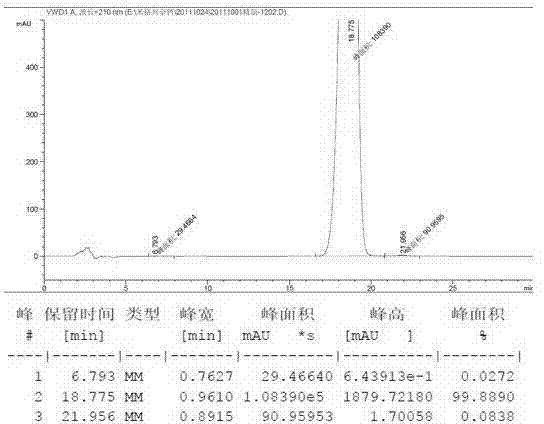
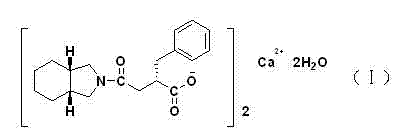
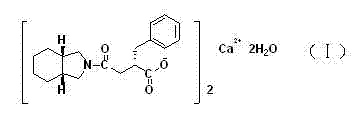
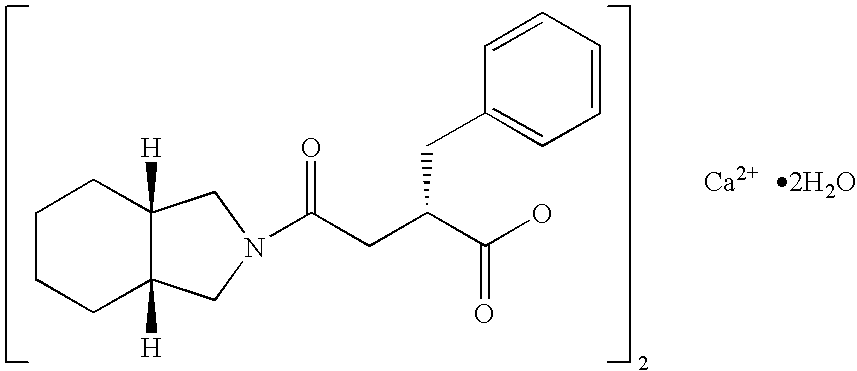
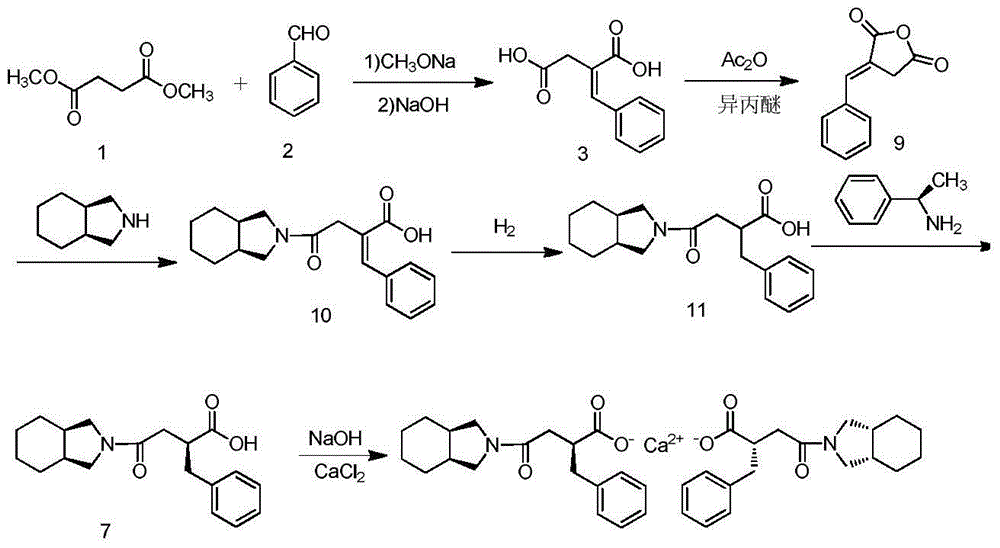
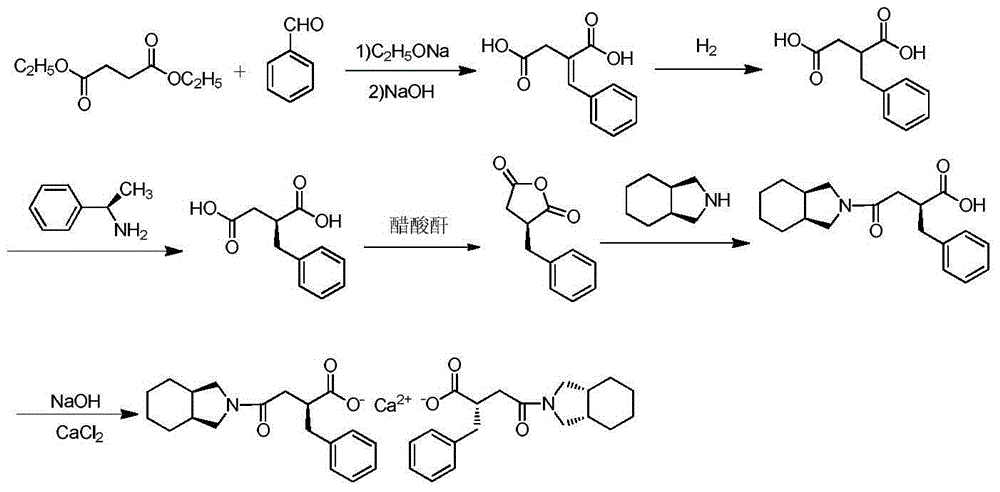
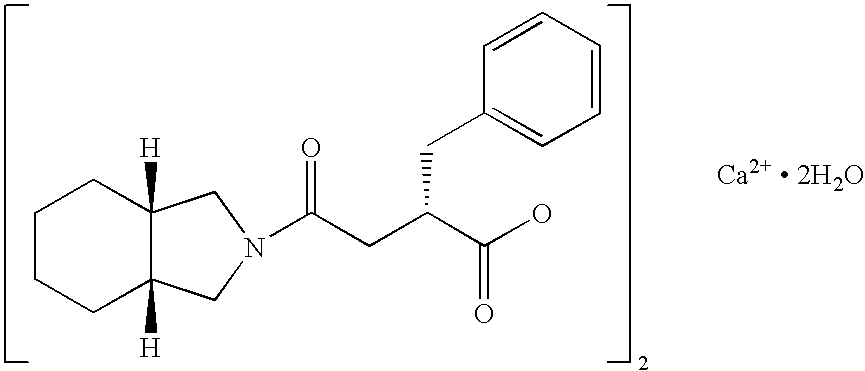
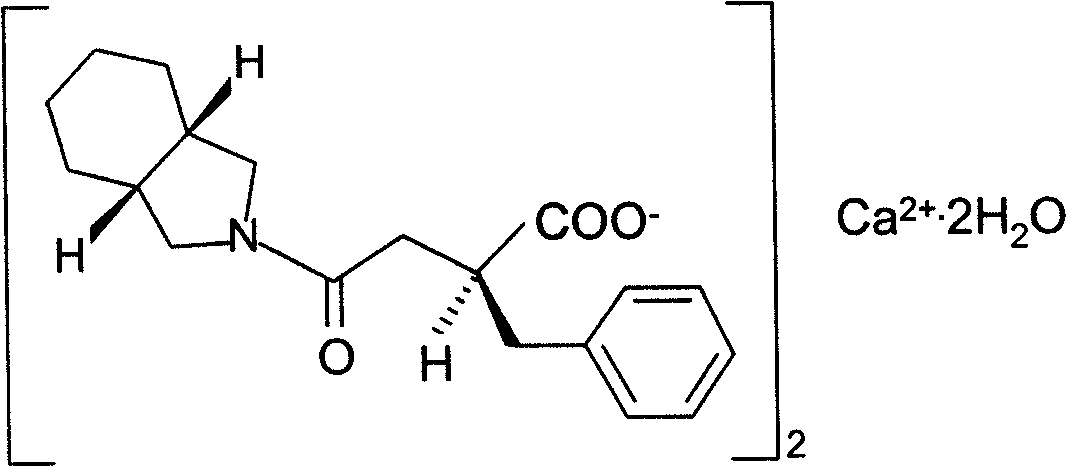
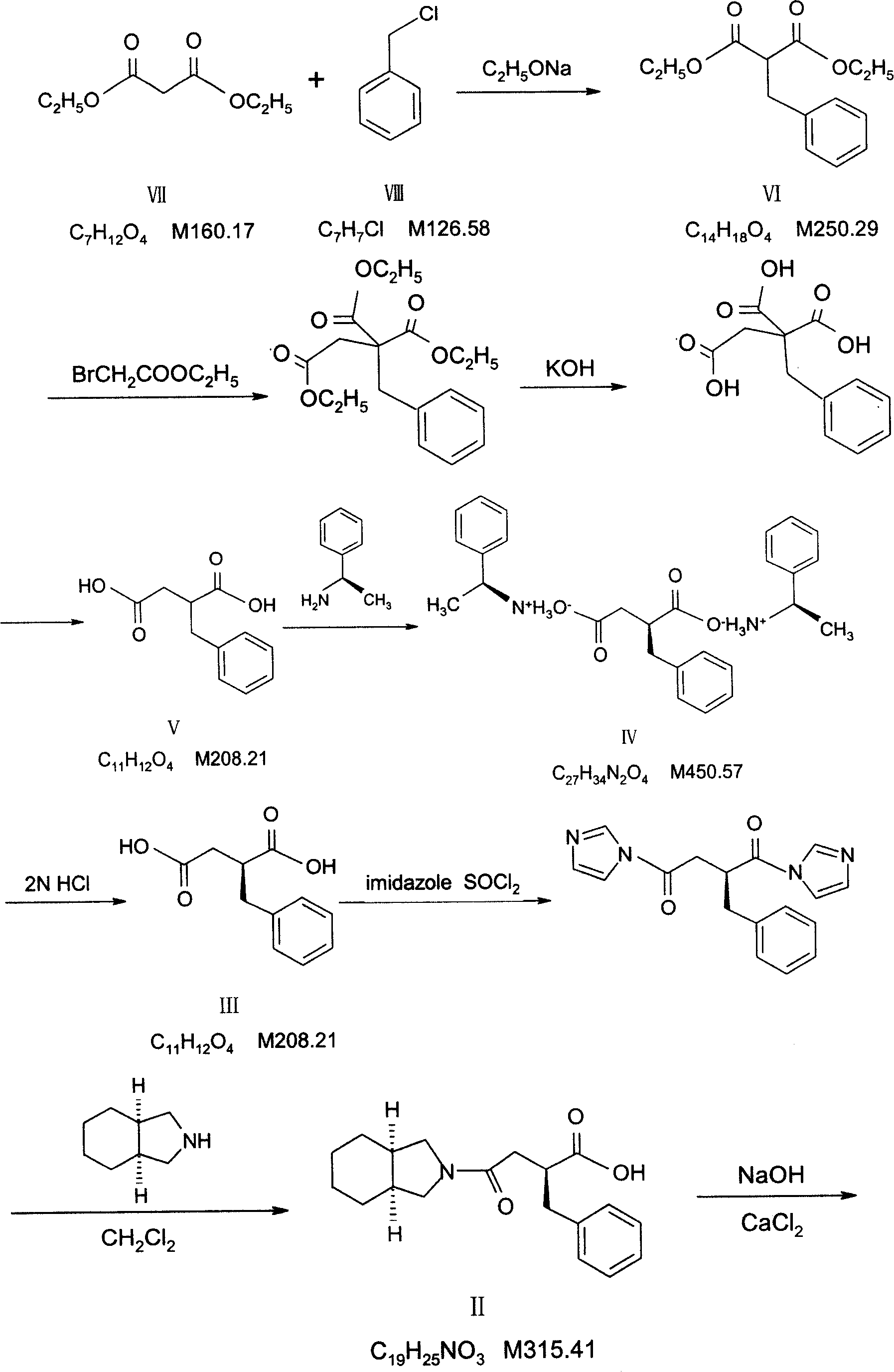
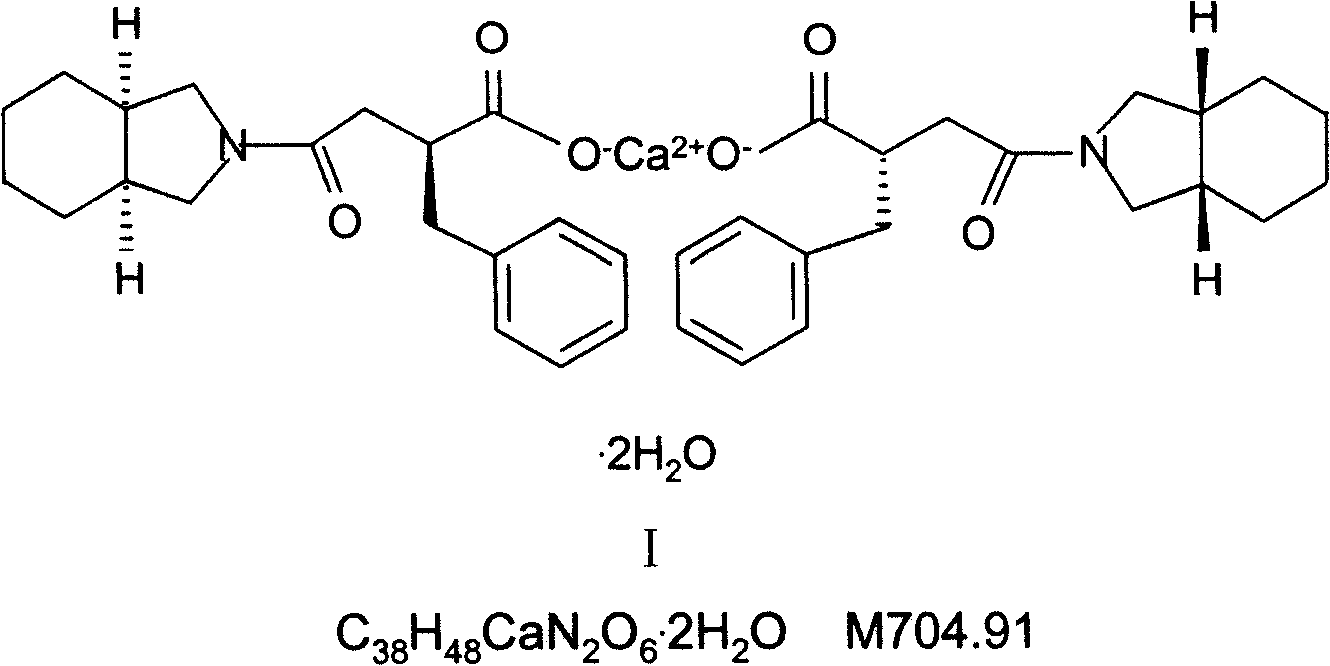
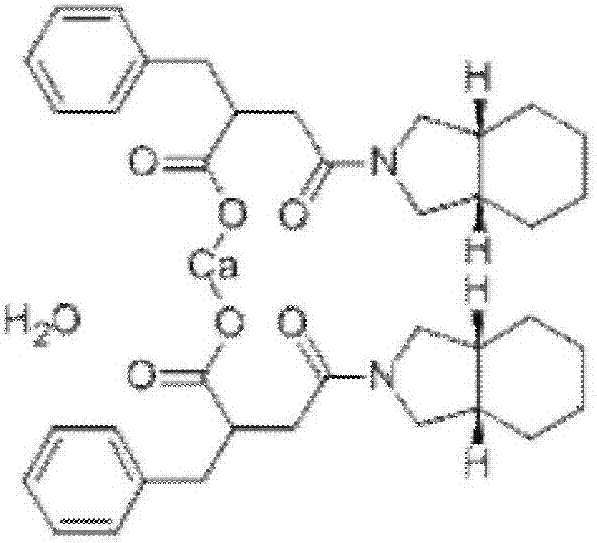
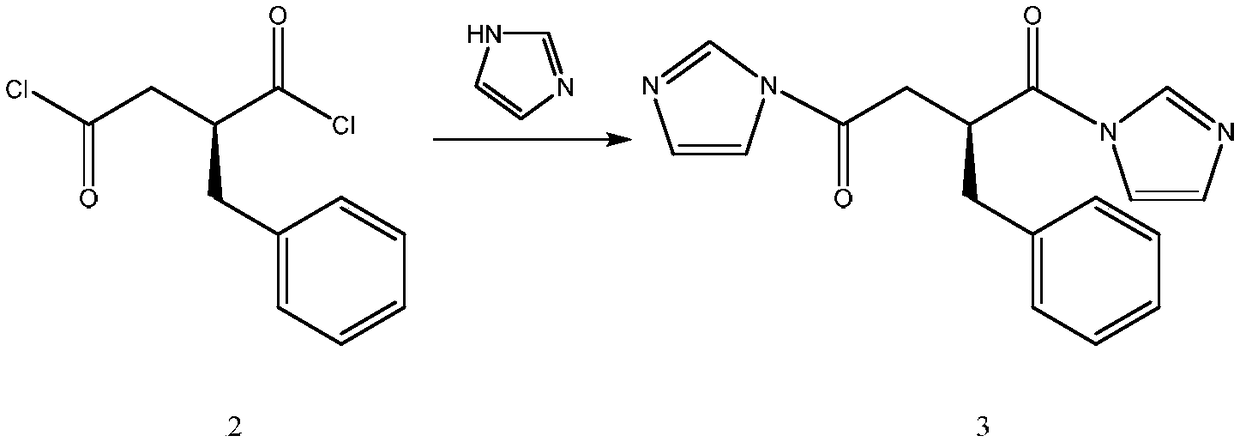
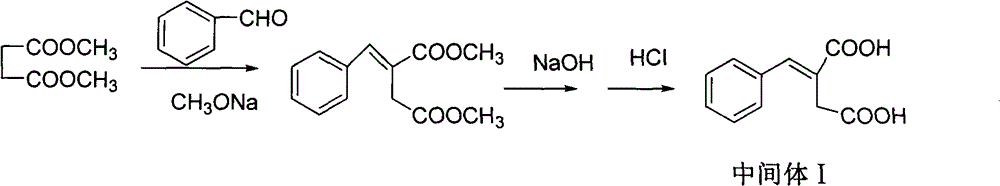
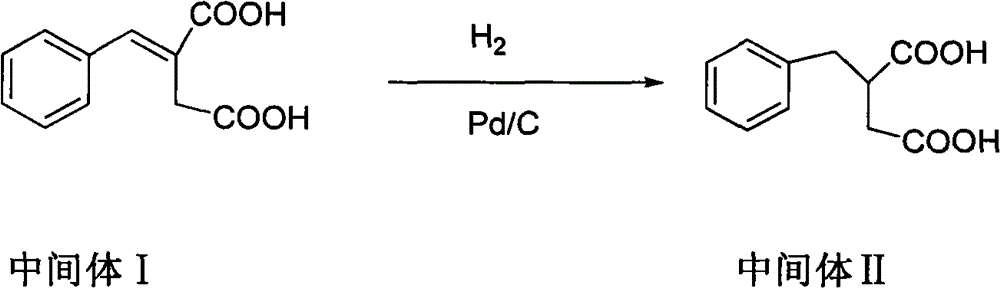
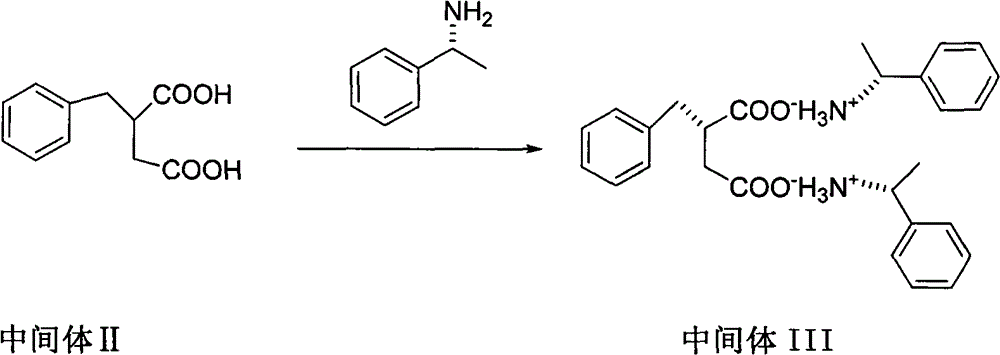
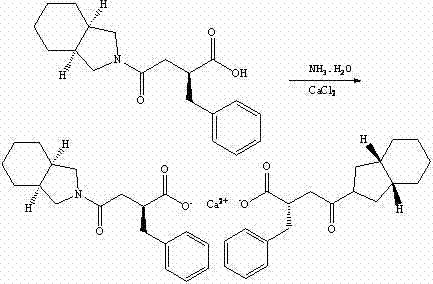
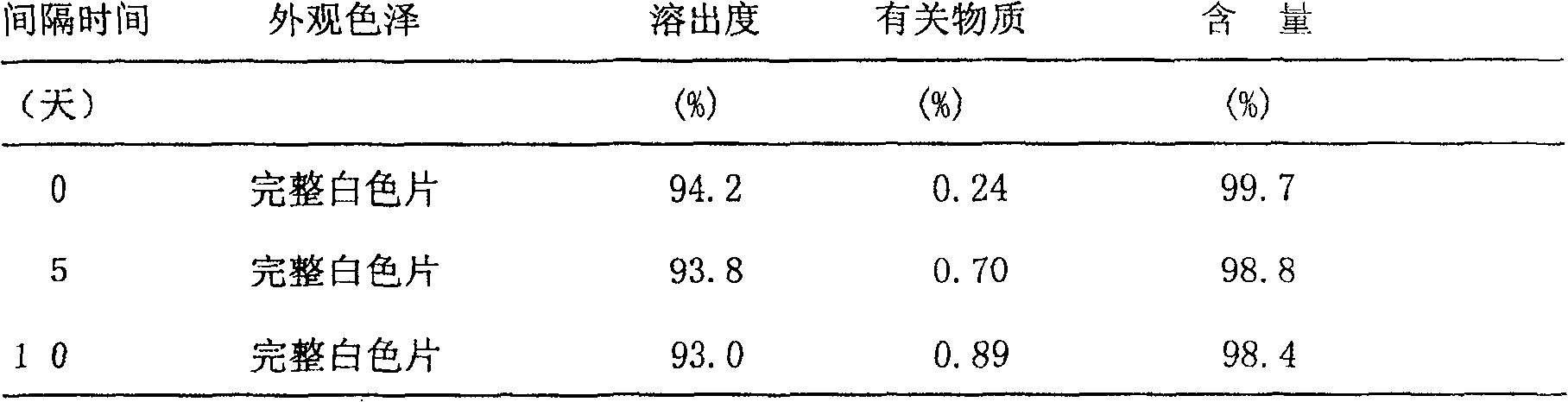
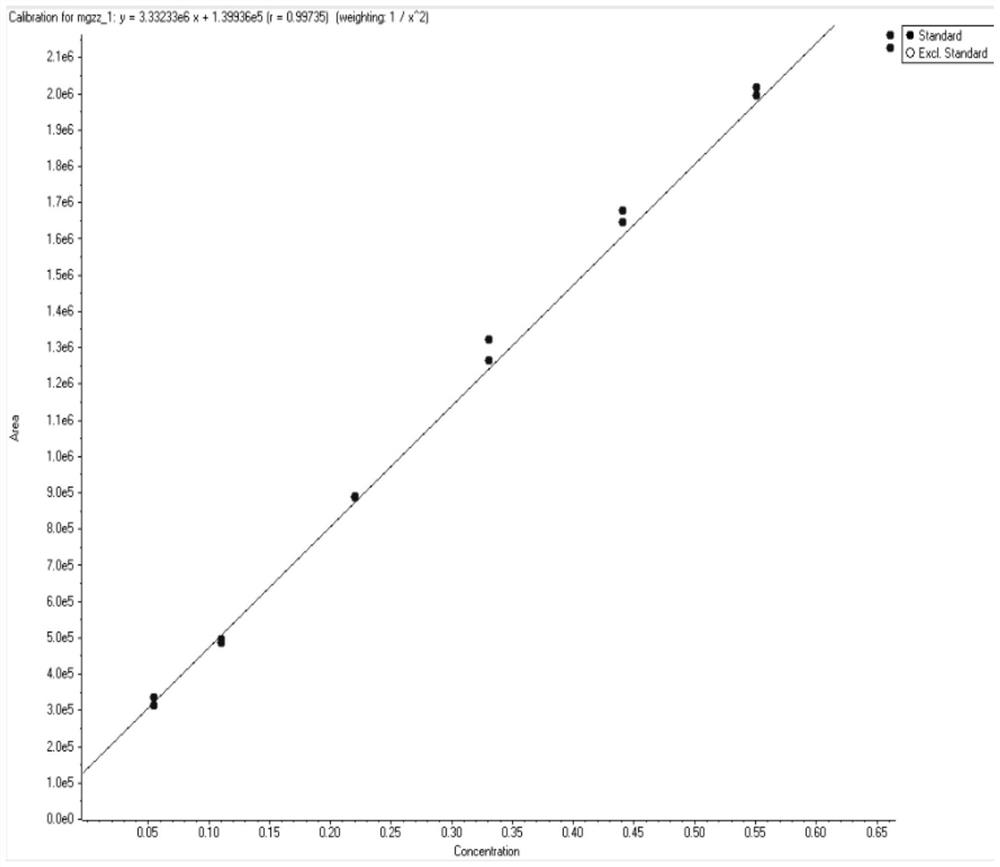
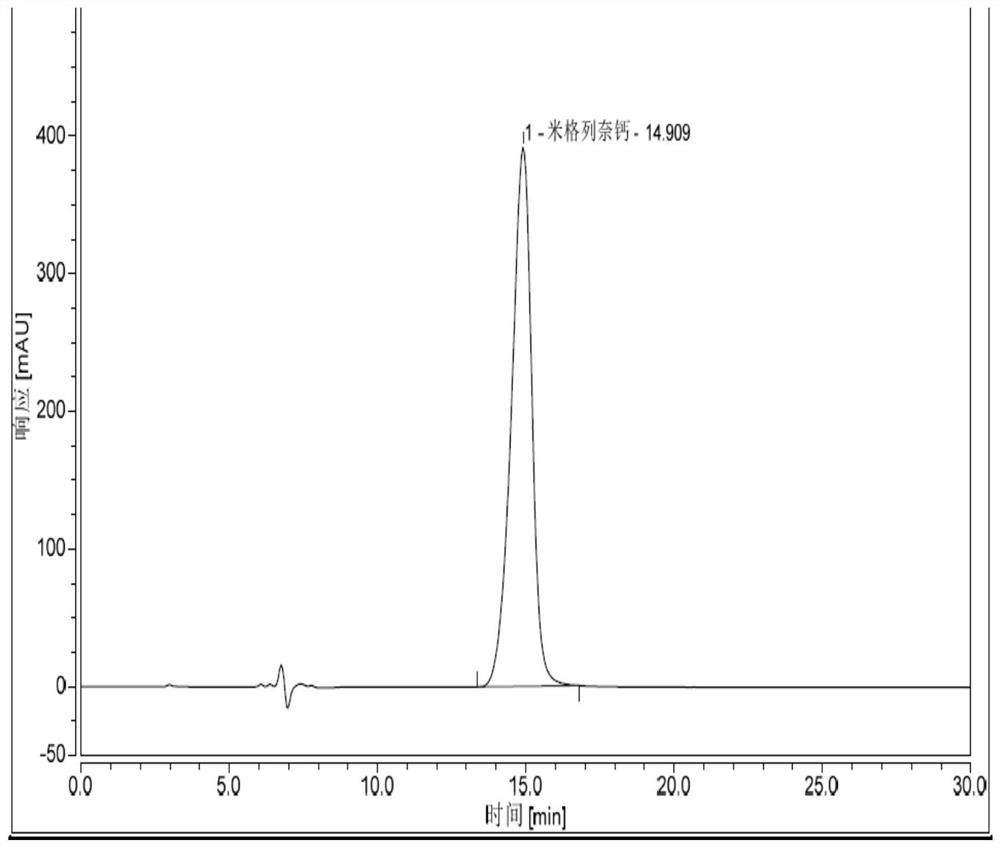
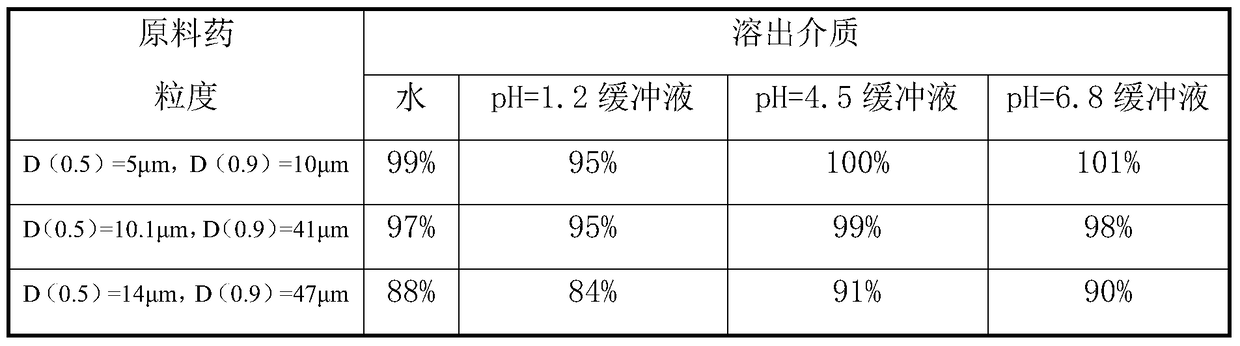
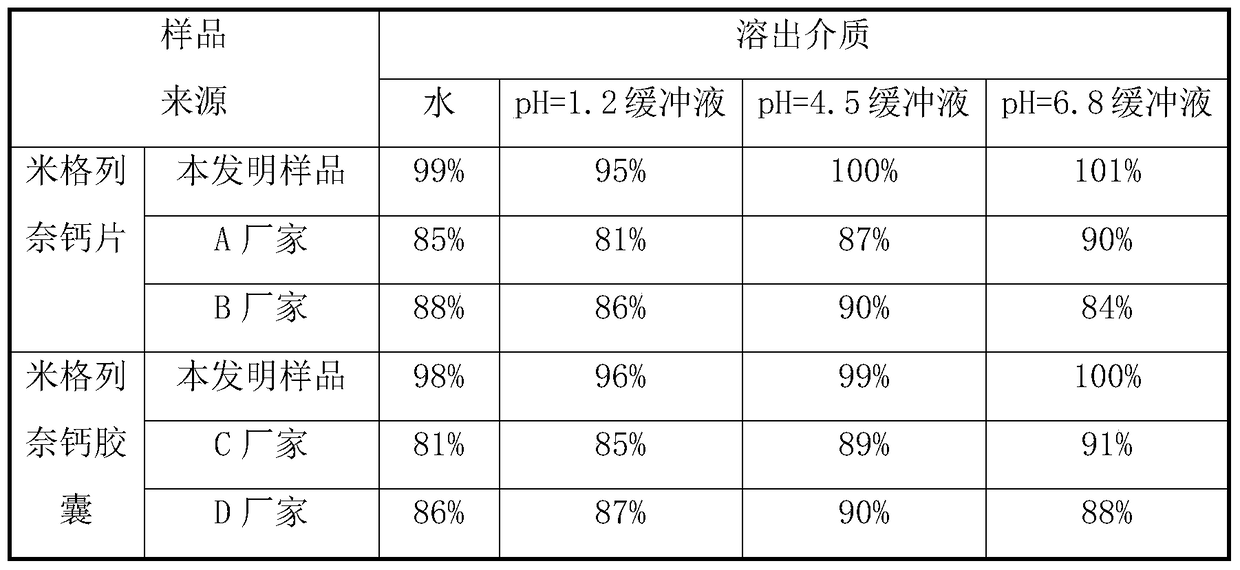
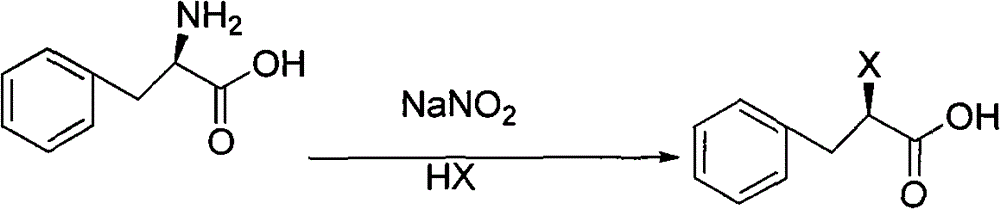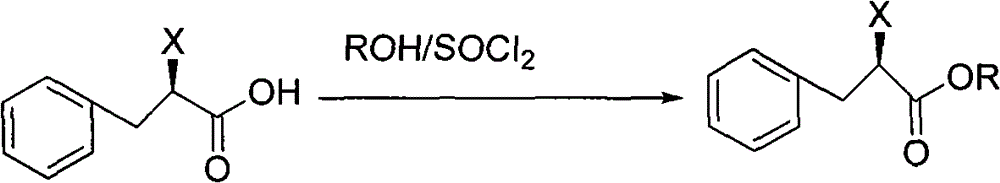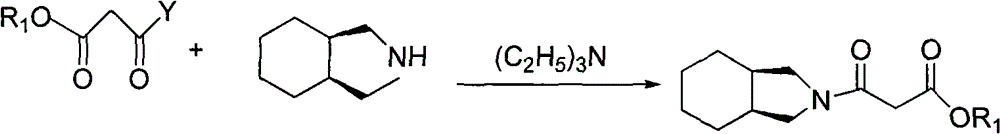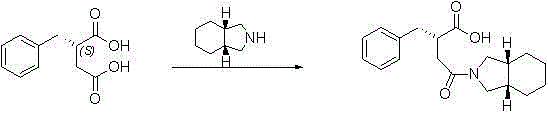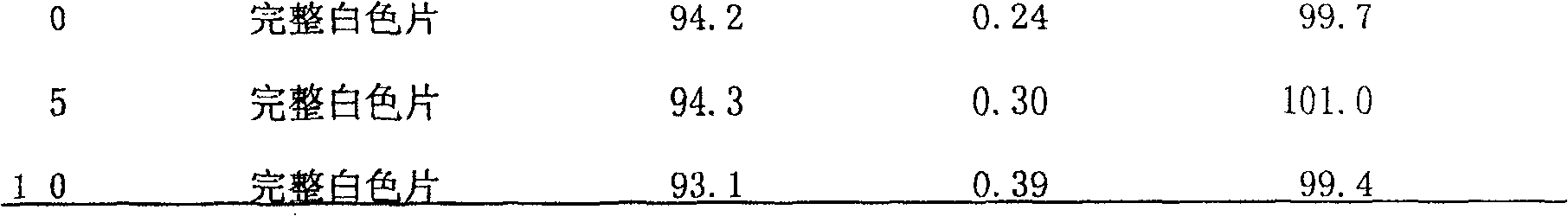Patents
Literature
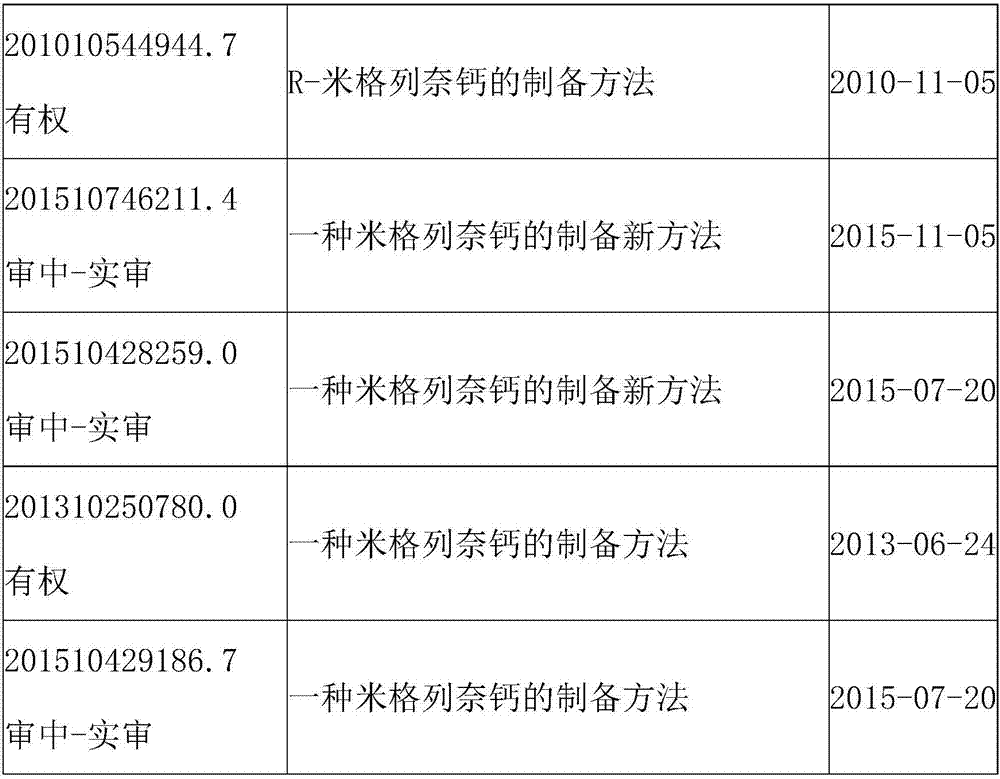
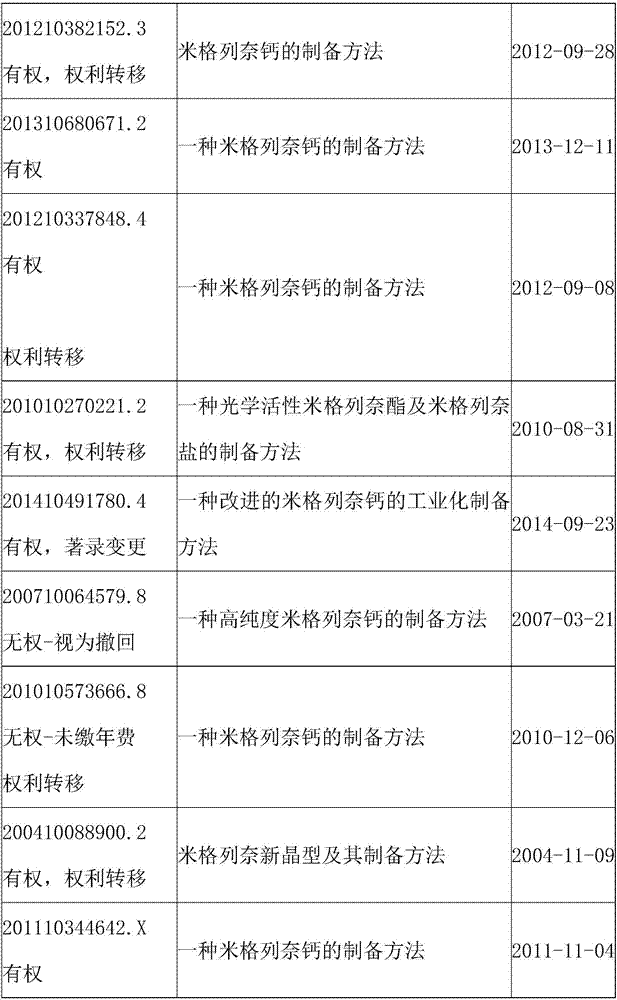
54 results about "MITIGLINIDE CALCIUM" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mitiglinide Calcium Hydrate is a medicine available in a number of countries worldwide. A list of US medications equivalent to Mitiglinide Calcium Hydrate is available on the Drugs.com website.
Drug composition for blood sugar control
InactiveUS20050267195A1Improve blood sugar controlInhibition is effectiveBiocideSenses disorderAcute hyperglycaemiaDisease
The present invention provides pharmaceutical compositions which can achieve good state of glycemic control and correct postprandial hyperglycemia and early morning fasting hyperglycemia. The present pharmaceutical composition is for administration before meal to control blood glucose, which comprises 5 to 45 mg, as a single dose, of mitiglinide or a pharmaceutically acceptable salt thereof, or a hydrate thereof (for example, mitiglinide calcium salt hydrate). And said compositions are extremely useful for prevention or treatment of, for example, type II diabetes, because the frequency of adverse drug reactions such as hypoglycemic symptoms and gastrointestinal disorders is low.
Owner:KISSEI PHARMA
Preparation of mitiglinide calcium and its quality control method
InactiveCN1844096AImprove accuracyHigh sensitivityOrganic active ingredientsOrganic chemistryPropanoic acidMalonate
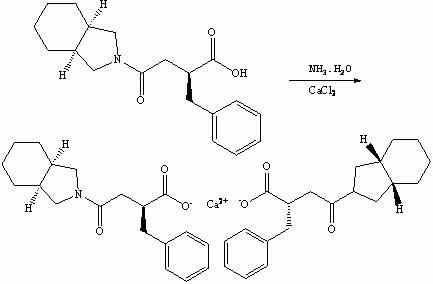
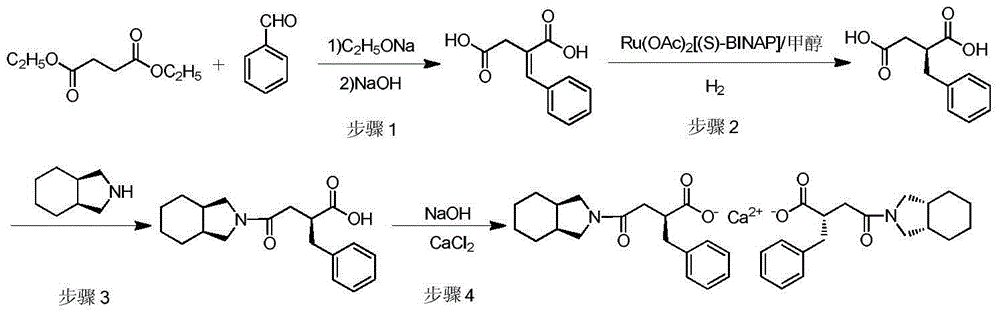
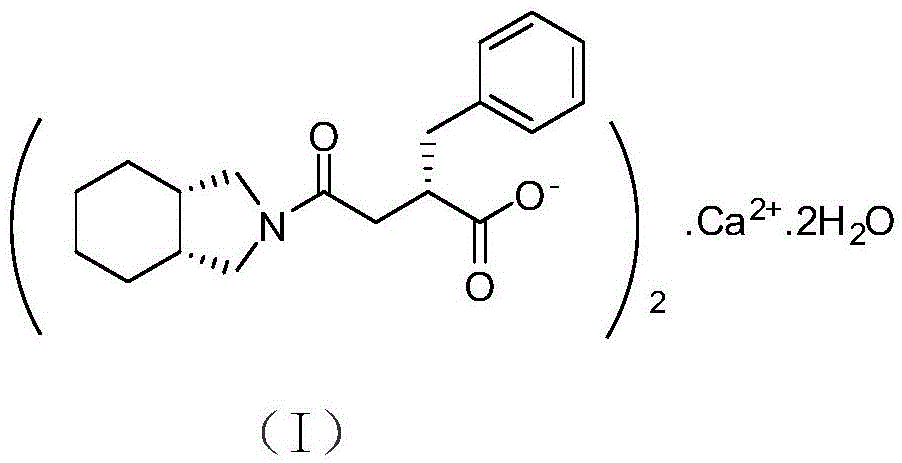
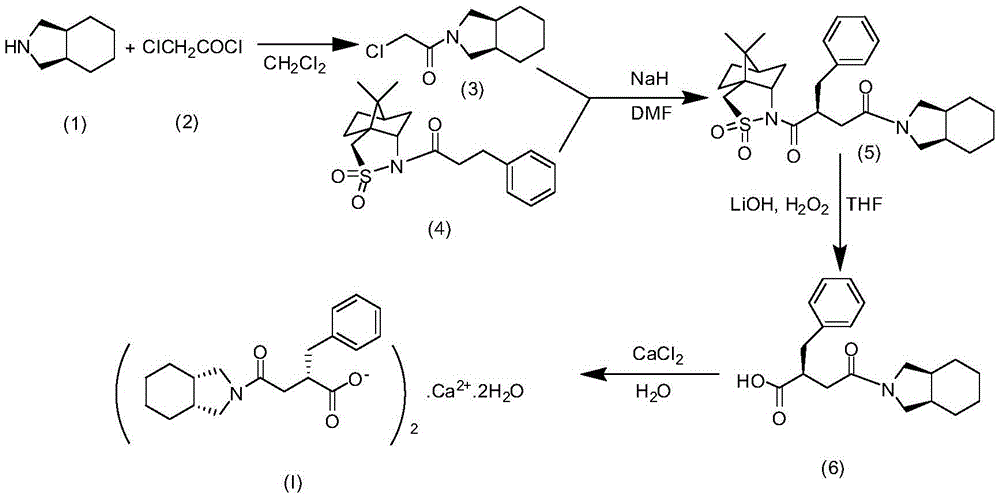
The invention relates to a preparation and quality control method of Mitiglinide Calcium comprising steps: 1,synthesis of cis- cyclohexyl-1,2-dimethylacid imide; 2,synthesis of cis-hexa-hydrogen isoindole; 3,synthesis of alpha-benzyldiethyl malonate; 4,synthesis of benzylsuccinic acid; 5,S- benzylsuccinic acid methylbenzylamine salt; 6,synthesis of s- benzylsuccinic acid; 7,synthesis of (2S)-2- benzyl-3-(cis-hexa-hydrogen isoindole-2- carbonyl) propanoic acid; 8,synthesis of Mitiglinide Calcium; 9,purity of Mitiglinide Calcium. This invention also contains the method for quality control of Mitiglinide Calcium comprising steps: watching deseription, messureing specific rotation, authenticating, checking, content messureing for Mitiglinide Calcium.
Owner:天津汉康医药生物技术有限公司
Preparation method of mitiglinide calcium
InactiveCN102101838ASave cis-hexahydroisoindoleHigh chiral separationOrganic chemistryAcetic anhydrideBenzaldehyde
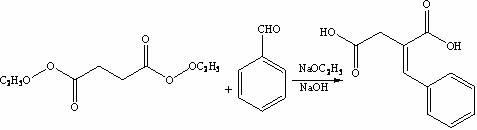
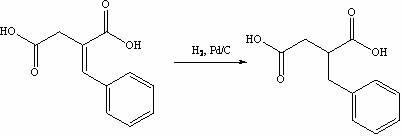
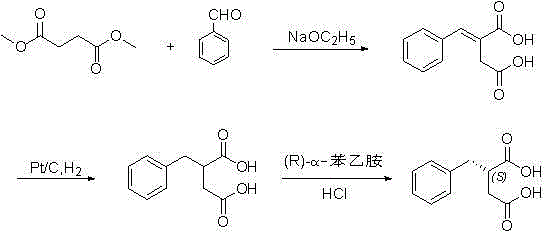
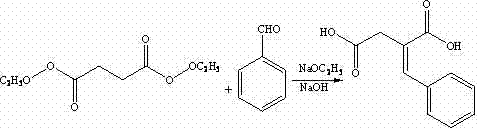
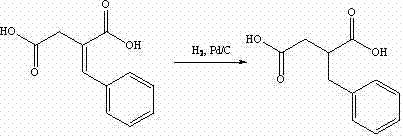
The invention discloses a preparation method of mitiglinide calcium. The method comprises the following steps of: performing Stobble condensation on diethyl succinate and benzaldehyde serving as raw materials in ethanol by using sodium alcoholate; hydrolyzing to obtain toluenyl butane diacid; performing catalytic hydrogenation on the toluenyl butane diacid to obtain DL-2-benzyl butane diacid; resolving the DL-2-benzyl butane diacid with (R)-chiral amine to obtain (S)-2-benzyl butane diacid; reacting the (S)-2-benzyl butane diacid under the action of acetic anhydride to obtain acid anhydride ; reacting the obtained acid anhydride with cis-hexahydroisoindole to obtain mitiglinide acid; and reacting the mitiglinide acid with calcium chloride and ammonia water to generate a mitiglinide calcium bihydrate. The preparation method of the mitiglinide calcium has the advantages of saving of cis-hexahydroisoindole serving as a raw material and high chiral separating degree.
Owner:周玉莲
Method for preparing high purity mitiglinide calcium
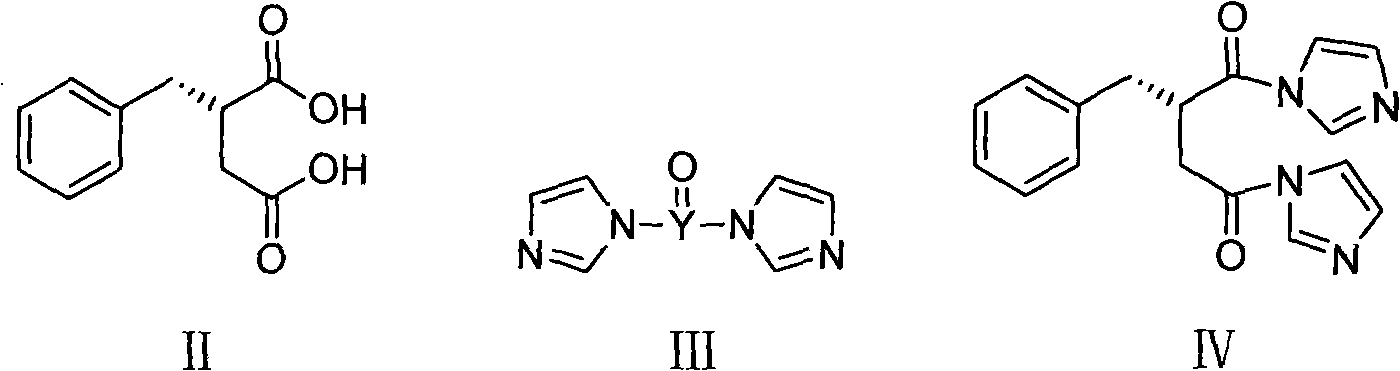
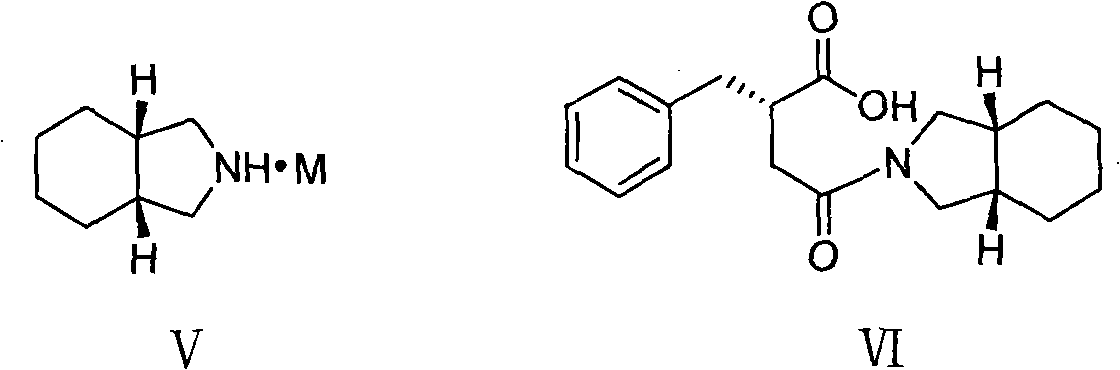
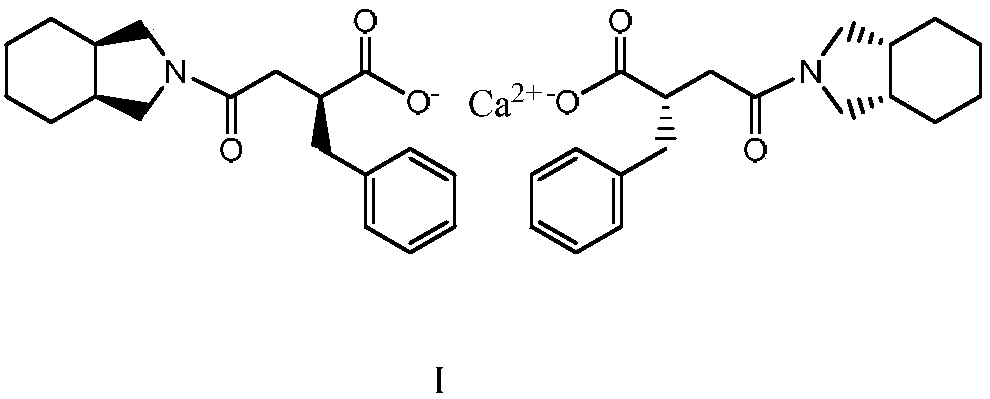
The present invention relates to a preparation method of high-purity mitiglinide calcium. A compound shown in the formula II and a compound shown in the formula V react under certain conditions to prepare the compound shown in the formula VI that reacts with calcium chloride to prepare the salt mitiglinide calcium.
Owner:BEIJING D VENTUREPHARM TECH DEV
Mitiglinide preparation and preparing method
InactiveCN1742721ALight weightYi FeiyangOrganic active ingredientsMetabolism disorderMethyl celluloseLactose
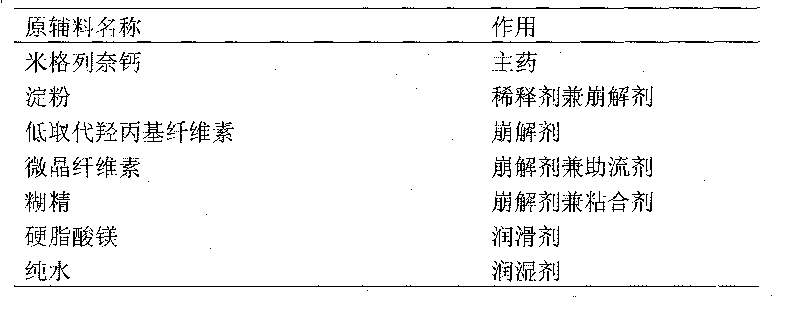
The present invention relates to a miglenei calcium preparation and its preparation method. Its composition includes (by weight portion) 5 portions of miglenei calcium, 5-10 portions of micropowder silicon gel, 20-30 portions of lactose, 15-30 portions of microcrystalline cellulose, 15-25 portions of starch, 5 portions of low-substituted hydroxypropyl cellulose and proper quantity of 2% hydroxypropyl methyl cellulose E15 solution as adhesive and proper quantity of water. Its preparation method includes the steps of sieving, mixing, granulating and after-treatment, etc.
Owner:周卓和
Preparation method of mitiglinide calcium
The invention discloses a preparation method of mitiglinide calcium, and the preparation method adopts (D)-phenylalanine as a raw material, and prepares mitiglinide calcium with a high yield through reactions of diazotization, hydroxy and carboxyl protection, nucleophilic substitution, hydrolysis, etc. The raw material used in the process provided by the invention has a wide source and a low price; the total yield is up to 47%; the optical purity is more than 99%; the reaction condition is mild; the reaction process is simple; disadvantages of low yield and long reaction steps of methods such as resolution, high pressure hydrogenation and the like reported in literature are avoided; and the invention provides a new selection for the preparation and production of mitiglinide calcium.
Owner:JIANGSU SIHUAN BIOENGINEERING PHARM CO LTD
Preparation method for Mitiglinide calcium
The invention relates to a preparation method for a bulk drug, i.e., Mitiglinide calcium, used for treating type II diabetes mellitus. According to the invention, improvement of the prior art is carried out: sodium methoxide is used to replace metallic sodium in the prior art, so reaction conditions are friendlier, and industrial production is easier to operate; the usage amount of benzaldehyde is reduced and argon protection is avoided, so environmental protection is benefited, and reaction yield is increased; and the usage amount of a palladium carbon (Pd / C) catalyst is reduced, which enables environmental pollution caused by excess usage of the heavy metal palladium to be reduced and cost to be lowered down.
Owner:DISHA PHARMA GRP +1
Preparation method of mitiglinide calcium
The invention relates to a preparation method of a bulk drug of mitiglinide calcium used for treating type 2 diabetes mellitus. The mitiglinide calcium is prepared by a series of reaction steps comprising using D-phenylalanine as a starting material, esterification and cis-hexahydroisoindoline reaction. In the reaction, the problem of high cost of special equipment for high pressure hydrogenation and operation as well as chiral catalyst in the traditional process can be solved, the use of chiral resolution and a resolving agent is avoided, and the yield is improved.
Owner:DISHA PHARMA GRP +1
Mitiglinide calcium dispersible tablet and preparation method thereof
InactiveCN101695481AQuick effectShort duration of actionOrganic active ingredientsMetabolism disorderMedicineHardness
The invention relates to a mitiglinide calcium dispersible tablet which is characterized by comprising the following materials parting parts by weight: 4-6 parts of mitiglinide calcium, 60-90 parts of starch, 12-18 parts of microcrystalline cellulose, 12.8-19.2 parts of dextrin, 0.8-1.2 parts of magnesium stearate and the balance of pure water. The invention has the advantages of rapid effect of blood sugar, short duration, capability of tabletting, good hardness and better disintegration.
Owner:江西中兴汉方药业有限公司
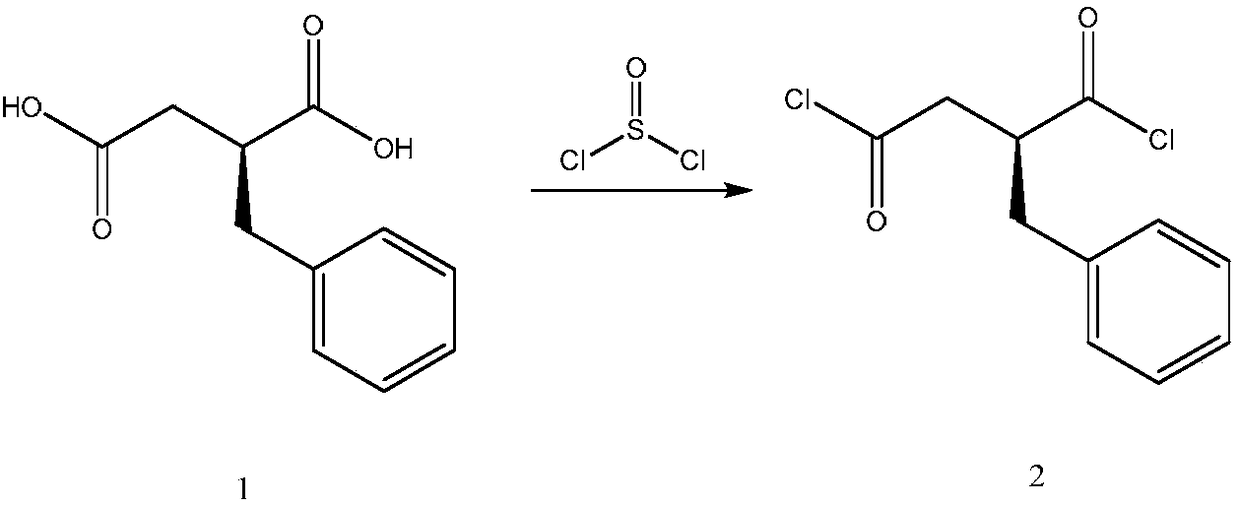
Preparation method of (S)-2-benzylsuccinic acid
ActiveCN105418401AHigh yieldReduce generationPreparation from carboxylic acid saltsOrganic compound preparationAlkaline waterBiochemical engineering
The invention provides a preparation method of (S)-2-benzylsuccinic acid, and relates to a method for preparing (S)-2-benzylsuccinic acid from (R)-2-benzylsuccinic acid. After EDTA is added in an alkaline aqueous solution containing the (R)-2-benzylsuccinic acid, racemization can be implemented effectively. By the method provided by the invention, the (S)-2-benzylsuccinic acid can be prepared from a solution of 2-benzylsuccinic acid (R)-alpha-phenylethanammonium generated in a production process of mitiglinide calcium, and economic benefit is improved further.
Owner:迪嘉药业集团股份有限公司
Rapid-dissolution mitiglinide preparation, and preparation method and detection method thereof
ActiveCN105560197ANot easy to produceReduce adverse effectsOrganic active ingredientsMetabolism disorderCarboxymethyl starchIn vitro digestion
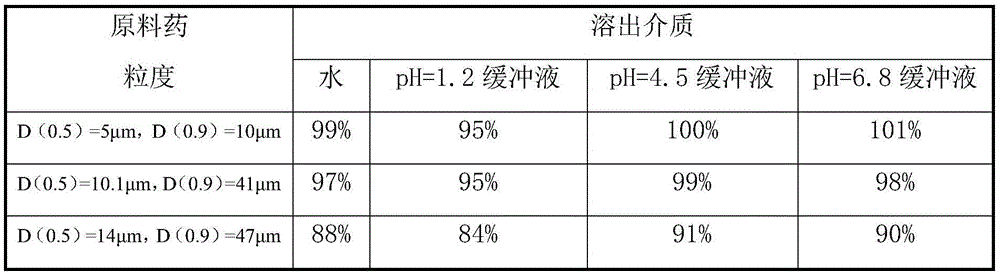
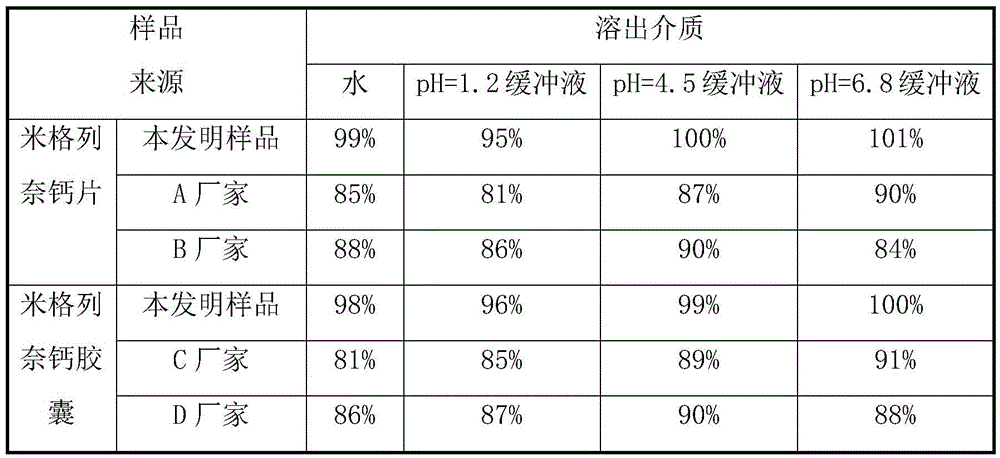
The invention relates to the field of medicinal preparations, and relates to a rapid-dissolution mitiglinide preparation, and a preparation method and a detection method thereof. The prescription of the preparation comprises 4-6 parts of a mitiglinide raw medicine, 25-45 parts of lactose, 15-35 parts of microcrystalline cellulose, 15-35 parts of starch, 1.5-5.5 parts of hydroxypropyl methylcellulose E3 or hydroxypropyl methylcellulose E5, 3-10 parts of sodium carboxymethyl starch and 0.05-0.3 parts of magnesium stearate. The rapid-dissolution mitiglinide preparation has the advantages of easy obtaining of auxiliary materials, low cost, simple preparation method, fast in vitro digestion, realization of the 10min dissolution rate of various dissolution media reaching 90% or above, and stable quality.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Preparation method of mitiglinide calcium
InactiveCN103450069AFew reaction stepsShort reaction timeOrganic chemistryChemical recyclingButanedioic acidPtru catalyst
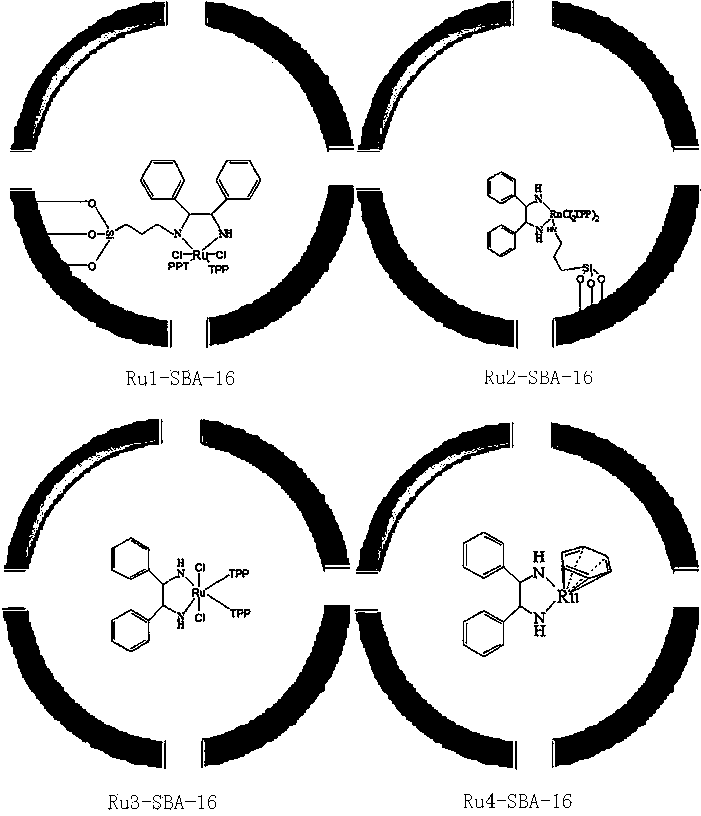
The invention discloses a preparation method of mitiglinide calcium. The preparation method comprises the following steps: (I) by taking diethyl succinate and benzaldehyde as raw materials, carrying out a reaction in a sodium ethoxide solution, and then, under the action of a multiphase asymmetric catalyst Ru-SBA-16, carrying out asymmetric hydrogenation to obtain a (S)-2-benzylsuccinic acid; (II) reacting the (S)-2-benzylsuccinic acid with perhydro-isoindole so as to generate mitiglinide; and (III) complexing the mitiglinide and calcium chloride so as to obtain a target compound mitiglinide calcium. According to the method, the reaction steps can be reduced, the reaction time can be shortened, and the chiral purity of mitiglinide calcium can be improved.
Owner:SHANXI DATONG UNIV
High purity mitiglinide calcium preparation method
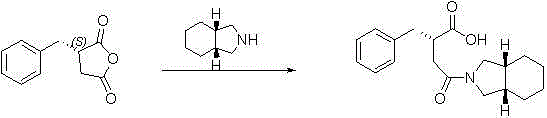
The invention discloses a high purity mitiglinide calcium preparation method including the following steps: synthesis of (2S)-2-benzyl-3-(cis-hexahydro isoindole-2-carbonyl) propionic acid; synthesis of (2S)-2-benzyl-3-(cis-hexahydro isoindole-2-carbonyl) benzyl propionate; hydrolysis of the (2S)-2-benzyl-3-(cis-hexahydro isoindole-2-carbonyl) benzyl propionate; and synthesis of mitiglinide calcium. The method simplifies synthetic route, and has the characteristics of convenient operation, low cost of raw materials, low equipment requirement, economy, environmental protection and simple technology. According to the method, the (2S)-2-benzyl-3-(cis-hexahydro isoindole-2-carbonyl) propionic acid can be crystallized after esterification, and is easy for purification and preservation and easy for realization of industrialized production. Tests confirm that the preparation method is easy in postprocessing, good in reaction area selectivity, high in product purity and relatively low in cost, and is conducive to industrial production.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Drug composition for prevention or inhibition of advance of diabetic complication
InactiveUS20090018181A1Prevent and inhibit progressionImprove blood sugar controlBiocideSenses disorderDiseaseAcute hyperglycaemia
Owner:KISSEI PHARMA
Improved mitiglinide calcium industrialized preparation method
InactiveCN104311471AAvoid the danger of flammable and explosive hydrogen gasIndustrial production safetyOrganic chemistrySuccinic acidButyric acid
The invention provides an improved mitiglinide calcium (I) preparation method, and is characterized in that the preparation method successively comprises the steps: step 1, preparation of 2-benzyl succinic acid; step 2, preparation of (S)-2-benzyl succinic acid; step 3, preparation of 2-(S)-benzyl-4-oxo-(cis-perhydroisoindole-2-yl)butyric acid; and step 4, preparation of mitiglinide calcium. The invention provides the mitiglinide calcium industrialized preparation method having the advantages of being economical and practical, simple to operate, short in reaction period and high in yield.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Detection method of impurity cis-octahydroisoindole in mitiglinide calcium
ActiveCN108982706AImprove medication safetyEasy to controlComponent separationIsoindolesBiochemistry
The invention discloses a detection method of impurity cis-octahydroisoindole in mitiglinide calcium. The detection method takes MG IIIC18 as a chromatographic column, and adopts an LC-MS / MS detectionmethod to conduct qualitative and quantitative analysis on cis-octahydroisoindole in mitiglinide calcium, and meanwhile, the methodology validation is conducted. The experiment proves that the methodhas the advantages of being high in specificity, rapid, sensitive and precise. The invention firstly builds up the quantitative and qualitative method of impurity cis-octahydroisoindole in mitiglinide calcium, the quality of mitiglinide calcium can be conveniently controlled, and accordingly the drug safety use of mitiglinide calcium is improved.
Owner:SHANDONG BOYUAN PHARM CO LTD
Detection method for mitiglinide calcium R-isomer
Owner:JIANGXI JINSHUIBAO PHARM CO LTD +1
Drug composition for prevention or inhibition of advance of diabetic complication
InactiveUS20050215607A1Improve blood sugar controlPrevent and inhibit progressionBiocideSenses disorderDiseaseAcute hyperglycaemia
The present invention provides pharmaceutical compositions which can achieve good state of glycemic control and correct postprandial hyperglycemia and early morning fasting hyperglycemia. The present pharmaceutical composition is for administration before meal to prevent or inhibit the progression of diabetic complication, which comprises 5 to 45 mg, as a single dose, of mitiglinide or a pharmaceutically acceptable salt thereof, or a hydrate thereof (for example, mitiglinide calcium salt hydrate). And said compositions are extremely useful for prevention or inhibition of progression of, for example, diabetic microvascular complications and arteriosclerotic diseases, because the frequency of adverse drug reactions such as hypoglycemic symptoms and gastrointestinal disorders is low.
Owner:KISSEI PHARMA
Preparation of mitiglinide calcium and its quality control method
The invention relates to a preparation and quality control method of Mitiglinide Calcium comprising steps: 1,synthesis of cis- cyclohexyl-1,2-dimethylacid imide; 2,synthesis of cis-hexa-hydrogen isoindole; 3,synthesis of alpha-benzyldiethyl malonate; 4,synthesis of benzylsuccinic acid; 5,S- benzylsuccinic acid methylbenzylamine salt; 6,synthesis of s- benzylsuccinic acid; 7,synthesis of (2S)-2- benzyl-3-(cis-hexa-hydrogen isoindole-2- carbonyl) propanoic acid; 8,synthesis of Mitiglinide Calcium; 9,purity of Mitiglinide Calcium. This invention also contains the method for quality control of Mitiglinide Calcium comprising steps: watching deseription, messureing specific rotation, authenticating, checking, content messureing for Mitiglinide Calcium.
Owner:天津汉康医药生物技术有限公司
Preparation method of mitiglinide calcium
InactiveCN107963989ALittle side effectsFew reaction stepsOrganic chemistryButanedioic acidIsoindoles
The invention relates to a preparation method of mitiglinide calcium, which includes steps of step 1, synthesis of benzal butanedioic acid; step 2, synthesis of benzylsuccinic acid; step 3, synthesisof (S)-benzylsuccinic acid (R)-alpha- phenylethylamine; step 4, synthesis of (S)-benzylsuccinic acid; step 5, synthesis of 2S-dihydro-isoindole indolebutyric acid; step 6, synthesis of crude product of mitiglinide calcium; step 7, refining of mitiglinide calcium.
Owner:JIANGXI JIMINKEXIN PHARMA +1
Method for preparing improved mitiglinide calcium
The invention relates to a method for preparing improved mitiglinide calcium. The method sequentially includes the steps of 1), preparation of acyl chloride; 2), preparation of active amide; 3), preparation of 2-(S)-benzyl-4-oxo-(cis-perhydroisindole-2-base) butyric acid; 4), preparation of the mitiglinide calcium. The method is concise in process, high in yield and low in production cost; compared with an existing acid anhydride process, the process applying the method has the advantages that product purity is remarkably improved, and easiness in industrialized production is achieved.
Owner:JIANGXI JIMINKEXIN PHARMA +1
A kind of preparation method of mitiglinide calcium
The invention relates to a preparation method for a bulk drug, i.e., Mitiglinide calcium, used for treating type II diabetes mellitus. According to the invention, improvement of the prior art is carried out: sodium methoxide is used to replace metallic sodium in the prior art, so reaction conditions are friendlier, and industrial production is easier to operate; the usage amount of benzaldehyde is reduced and argon protection is avoided, so environmental protection is benefited, and reaction yield is increased; and the usage amount of a palladium carbon (Pd / C) catalyst is reduced, which enables environmental pollution caused by excess usage of the heavy metal palladium to be reduced and cost to be lowered down.
Owner:DISHA PHARMA GRP +1
A kind of preparation method of mitiglinide calcium
InactiveCN103724253BRaw materials are easy to getMild reaction conditionsOrganic chemistryHydrolysisMITIGLINIDE CALCIUM
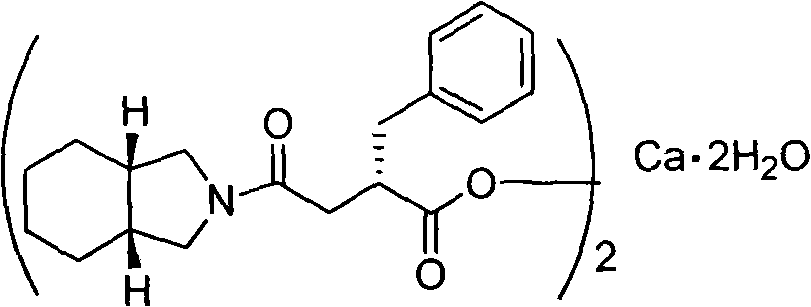
The invention provides a novel preparation method for Mitiglinide calcium hydrate. The method comprises the following steps: adopting cis-hexahydroisoindole as a raw material, conducting acylation reaction, hydrocarbonylation reaction, hydrolysis reaction, and salt forming reaction on the raw material, and obtaining the Mitiglinide calcium hydrate; a one-port process method is adopted for conducting the hydrocarbonylation reaction and the hydrolysis reaction, and the product purity can reach over 99.8%, and the yield can reach over 36%. The preparation method for Mitiglinide calcium hydrate has the advantages of the easy-to-get raw material, mild reaction condition, simple and practicable operation, and relatively high yield, therefore, the method has relatively higher industrial production value, and industrialization has been realized at present.
Owner:中国人民解放军联勤保障部队第九六六医院
Preparation method of mitiglinide calcium
InactiveCN102101838BSave cis-hexahydroisoindoleHigh chiral separationOrganic chemistryAcetic anhydrideBenzaldehyde
The invention discloses a preparation method of mitiglinide calcium. The method comprises the following steps of: performing Stobble condensation on diethyl succinate and benzaldehyde serving as raw materials in ethanol by using sodium alcoholate; hydrolyzing to obtain toluenyl butane diacid; performing catalytic hydrogenation on the toluenyl butane diacid to obtain DL-2-benzyl butane diacid; resolving the DL-2-benzyl butane diacid with (R)-chiral amine to obtain (S)-2-benzyl butane diacid; reacting the (S)-2-benzyl butane diacid under the action of acetic anhydride to obtain acid anhydride ;reacting the obtained acid anhydride with cis-hexahydroisoindole to obtain mitiglinide acid; and reacting the mitiglinide acid with calcium chloride and ammonia water to generate a mitiglinide calcium bihydrate. The preparation method of the mitiglinide calcium has the advantages of saving of cis-hexahydroisoindole serving as a raw material and high chiral separating degree.
Owner:周玉莲
Mitiglinide calcium formulation and its detection method
The invention relates to a new formulation of mitiglinide calcium and its quality control method. The pharmaceutical composition of the invention is granulated by mixing mitiglinide calcium with lactose, mannitol, microcrystalline cellulose and sodium carboxymethyl starch Finally, the hygroscopicity is greatly reduced, the fluidity is increased, the dissolution is rapid, and the stability of the product is enhanced.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Method for preparing hypoglycemic drug mitiglinide calcium
The invention relates to a method for preparing a hypoglycemic drug mitiglinide calcium. According to the method, under the temperature of -5 DEG C-10 DEG C, 30-60g of 2-benzylidene-3-(cis-perhydro-isoindolyl-2-carbonyl)propionic acid is dissolved in 200-500 milliliters of a reaction solvent, 2-5 g of a S-configuration aminoalcohol catalyst, 8-15 g of a borohydride and 15-25 g of trimethylchlorosilane are added, the materials are stirred for a reaction for 4-8 hours, pressure reduction is carried out for steaming the solvent, 400-600 milliliters of water and 4-6 milliliters of hydrochloric acid are added, ethyl acetate is used for extraction, an extract is merged, the extract is dried and concentrated to obtain a concentrate, the concentrate is dissolved in 400-600 mL of ethanol with mass concentration of 75-95%, 3-5 g of sodium hydroxide is added, after stirring and dissolving the materials, 8-12 g of calcium chloride is added, precipitate is obtained, and then the precipitate is subjected to pumping filtration to obtain mitiglinide calcium. The preparing method has the advantages of simple process, energy saving and environmental protection, can greatly increase the overall yield and obviously reduce the production cost, is easy to industrial production, is an innovation of a medicine for treating diabetes, and has large economic and social benefits.
Owner:HENAN UNIV OF CHINESE MEDICINE
A method for detecting impurity cis-perhydroisoindole in mitiglinide calcium
ActiveCN108982706BImprove medication safetyEasy to controlComponent separationPhysical chemistryChromatography column
Owner:SHANDONG BOYUAN PHARM CO LTD
A kind of detection method of mitiglinide calcium r-isomer
Owner:JIANGXI JINSHUIBAO PHARM CO LTD +1
A fast-dissolving mitiglinide calcium preparation and its preparation and detection method
ActiveCN105560197BNot easy to produceReduce adverse effectsOrganic active ingredientsMetabolism disorderCarboxymethyl starchIn vitro digestion
The invention relates to the field of medicinal preparations, and relates to a rapid-dissolution mitiglinide preparation, and a preparation method and a detection method thereof. The prescription of the preparation comprises 4-6 parts of a mitiglinide raw medicine, 25-45 parts of lactose, 15-35 parts of microcrystalline cellulose, 15-35 parts of starch, 1.5-5.5 parts of hydroxypropyl methylcellulose E3 or hydroxypropyl methylcellulose E5, 3-10 parts of sodium carboxymethyl starch and 0.05-0.3 parts of magnesium stearate. The rapid-dissolution mitiglinide preparation has the advantages of easy obtaining of auxiliary materials, low cost, simple preparation method, fast in vitro digestion, realization of the 10min dissolution rate of various dissolution media reaching 90% or above, and stable quality.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Miglinide calcium composition tablet and preparation method thereof
ActiveCN103565764BFast disintegrationHigh dissolution rateOrganic active ingredientsMetabolism disorderLactoseMagnesium stearate
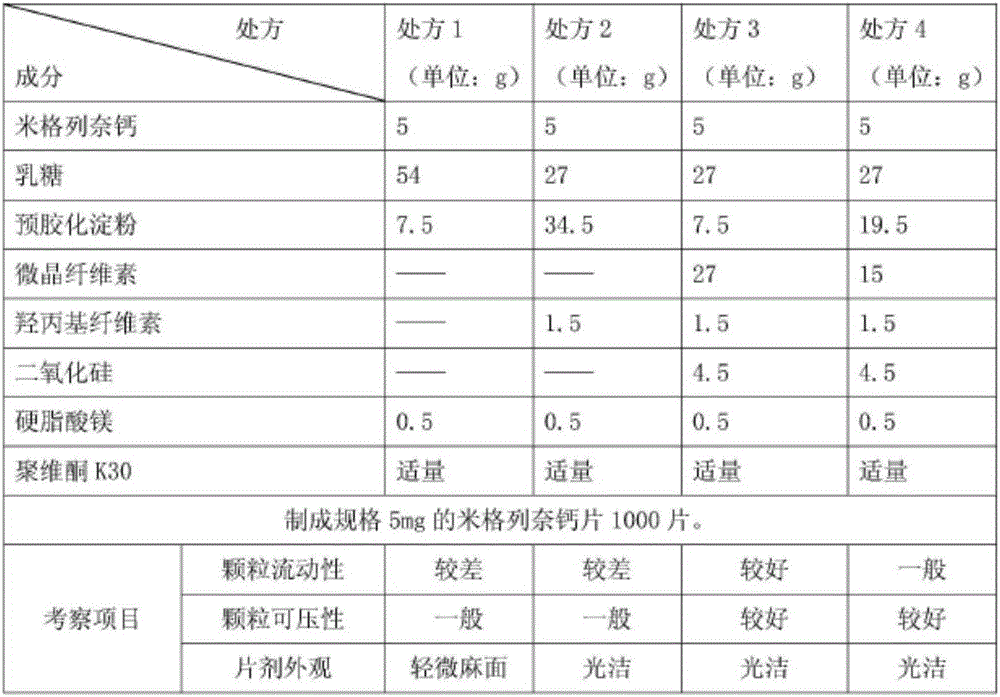
The invention provides a mitiglinide calcium composition tablet and a preparation method thereof. The weight ratio of the composition components is: 7-12 parts of mitiglinide calcium, 40-70 parts of lactose, and 5-5 parts of pregelatinized starch 20 parts, 25-60 parts of microcrystalline cellulose, 1-5 parts of hydroxypropyl cellulose, 5-12 parts of silicon dioxide, 0.1-3 parts of magnesium stearate, appropriate amount of povidone K30. The composition tablet of mitiglinide calcium provided by the present invention has components with readily available synthetic raw materials and low cost. The preparation method is simple, and the prepared tablet has the characteristics of fast disintegration speed, high dissolution rate, good stability, accurate dosage, convenient taking, and portability. The process of the invention is simple, easy to control, short production cycle, and low production cost. Suitable for industrial production.
Owner:NANHAI PHARMA CHONGQING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com