A fast-dissolving mitiglinide calcium preparation and its preparation and detection method
A mitiglinide calcium preparation technology, which is applied in the field of fast-dissolving mitiglinide calcium preparations and its preparation and testing, can solve the problems of affecting patient confidence, high failure rate, weight gain, etc., and avoid granulation time And the stirring speed is too low, it is not easy to produce dust, and the effect of uniform properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 A fast-dissolving mitiglinide calcium tablet
[0032] 5 parts of mitiglinide calcium; 40 parts of lactose; 30 parts of microcrystalline cellulose; 20 parts of starch; 55 parts of hypromellose E; 5 parts of carboxymethyl starch; 0.1 part of magnesium stearate.
[0033] The particle size of the mitiglinide calcium bulk drug D(0.5)≤10 μm, D(0.9)≤40 μm.
[0034] Prepare the method for the described mitiglinide calcium preparation of right, described method comprises the following steps:
[0035] 1) Miglinide calcium and lactose were mixed for 10 minutes to obtain material 1;
[0036] 2) Material 1 was put into a wet mixing granulator together with lactose, microcrystalline cellulose, starch, and 30% sodium carboxymethyl starch, and mixed at a stirring speed of 120 rpm / min for 8 minutes to obtain material 2;
[0037] 3) Prepare hypromellose E3 or E5 into an aqueous solution with a concentration of 1%-10%, add material 2 to it, and granulate to obtain material 3. ...
Embodiment 2
[0042] Example 2 A fast-dissolving mitiglinide calcium capsule
[0043] A mitiglinide calcium preparation, characterized in that the preparation comprises the following components in parts by weight:
[0044] 5 parts of mitiglinide calcium; 30 parts of lactose; 20 parts of microcrystalline cellulose; 30 parts of starch; 1.5 parts of sodium carboxymethyl starch; 32 parts of hypromellose E; 4 parts of sodium carboxymethyl starch; 0.1 part of magnesium stearate.
[0045] The particle size of the mitiglinide calcium bulk drug D(0.5)≤10 μm, D(0.9)≤40 μm.
[0046] Prepare the method for the described mitiglinide calcium preparation of right, described method comprises the following steps:
[0047] 1) mitiglinide calcium and lactose were mixed for 5 minutes to obtain material 1;
[0048] 2) Material 1 was put into a wet mixing granulator together with lactose, microcrystalline cellulose, starch, and 40% sodium carboxymethyl starch, and mixed at a stirring speed of 100 rpm / min for ...
Embodiment 3
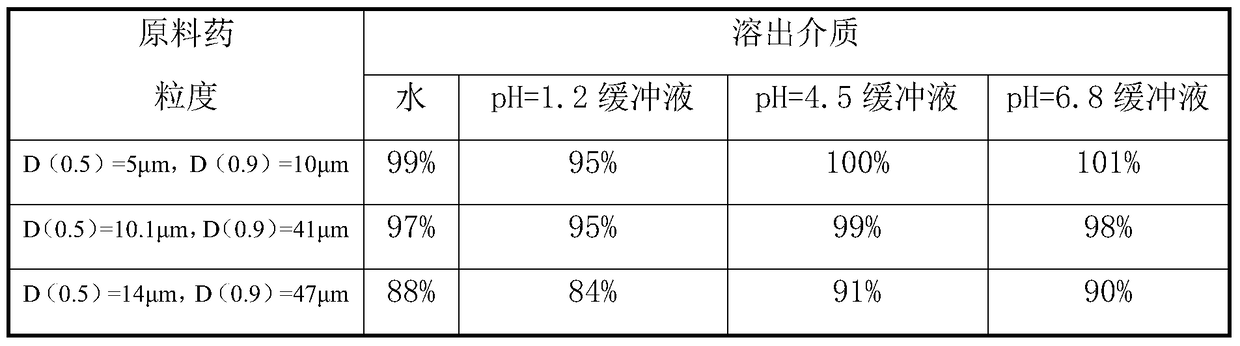
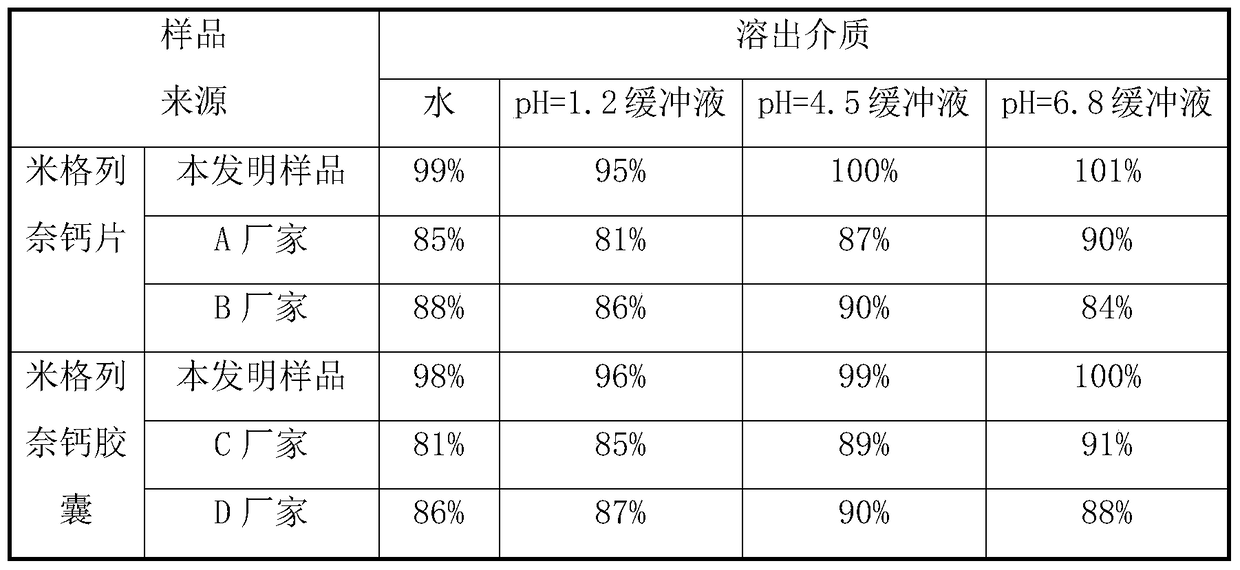
[0054] Embodiment 3, different granularity mitiglinide calcium crude drug preparation mitiglinide calcium tablet dissolution rate influence
[0055] Adopt the method of the present invention, other raw material proportioning and method are consistent, compare the impact of different raw material particle sizes on the dissolution rate of mitiglinide calcium tablets, the specific results are shown in Table 1 (slurry method, 50rpm, 900ml, n=6).
[0056] Table 1 Effect of different particle sizes of mitiglinide calcium raw materials on the dissolution rate of mitiglinide calcium tablets
[0057]
[0058] As can be seen from Table 1, the particle size of the mitiglinide calcium raw material has a significant impact on the dissolution rate of the mitiglinide calcium tablet. The dissolution rate of mitiglinide calcium tablets can reach more than 90% in 10 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com