Miglinide calcium composition tablet and preparation method thereof
A technology of mitiglinide calcium and its composition, which is applied in the field of pharmaceutical preparations, can solve problems such as unstable storage at room temperature, low solubility in water, and slow dissolution of tablets, and achieve accurate dosage, short production cycle, and fast disintegration speed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The preparation of embodiment 1 mitiglinide calcium composition tablet
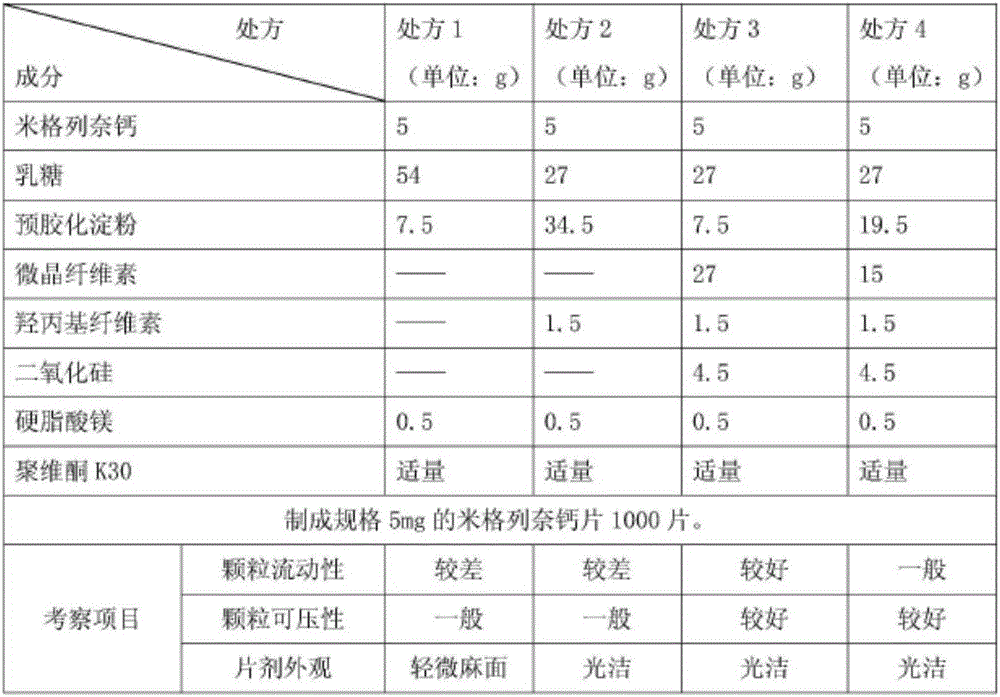
[0067] Prepare three batches of pilot test samples according to the above-mentioned determined prescription process, summarized as follows:
[0068] Feeding and output (5mg) of the sample in table 2
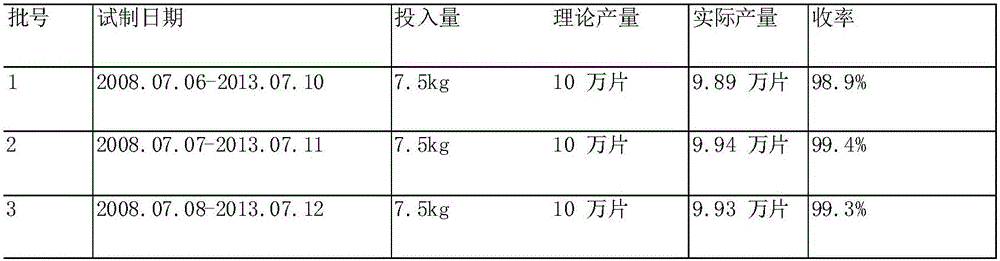
[0069] Batch number, date of trial production, main drug dosage, theoretical output and output.
[0070]
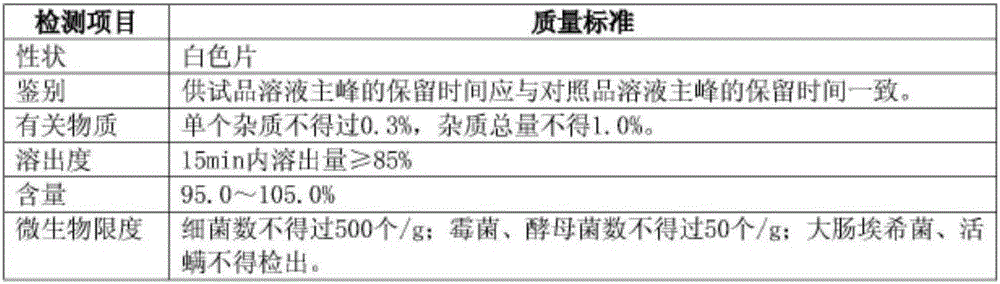
[0071] Table 3 Sample Testing Standards
[0072]
[0073] Reference substance information:
[0074] Lot No. 13-SSR-75-1; Quantity 98%; Source Toronto Research Chemicals Inc.
[0075] Table 4 Sample Test Results
[0076]
[0077] It can be seen from the above that all indicators of the samples prepared by this prescription process all meet the requirements, so the process is feasible.
Embodiment 2
[0078] Embodiment 2: Preparation of mitiglinide calcium composition tablet
[0079] Produce 1000 pieces of mitiglinide calcium tablets (specification 5mg), and the core contains the following raw materials in parts by weight: mitiglinide calcium 5g, lactose 30g, pregelatinized starch 7.5g, microcrystalline cellulose 30g, hydroxypropyl cellulose 1.5g, 4.5g silicon dioxide, 0.5g magnesium stearate, appropriate amount of povidone K30.
[0080] Pass mitiglinide calcium through a 100-mesh sieve, pass lactose, pregelatinized starch, microcrystalline cellulose, hydroxypropyl cellulose, and silicon dioxide through a 80-mesh sieve respectively; Calcium glinide, lactose, pregelatinized starch, microcrystalline cellulose, hydroxypropyl cellulose, and silicon dioxide are evenly mixed to obtain the first mixture; add 5% povidone k30 aqueous solution to the first mixture to make soft material, passed through a 24-mesh sieve for granulation, dried the obtained granules at 60°C until the wat...
Embodiment 3
[0081] The preparation of embodiment 3 mitiglinide calcium composition tablet
[0082] Production of 1000 mitiglinide calcium tablets (specification 10mg) tablet cores contains the following raw materials in parts by weight: mitiglinide calcium 10g, lactose 60g, pregelatinized starch 15g, microcrystalline cellulose 55g, hydroxypropyl cellulose 3g, Silicon dioxide 9g, magnesium stearate 1g, appropriate amount of povidone K30.
[0083] Pass mitiglinide calcium through a 100-mesh sieve, pass lactose, pregelatinized starch, microcrystalline cellulose, hydroxypropyl cellulose, and silicon dioxide through a 80-mesh sieve respectively; Calcium linide, lactose, pregelatinized starch, microcrystalline cellulose, hydroxypropyl cellulose, and silicon dioxide are uniformly mixed to obtain the first mixture; 5% povidone k30 aqueous solution is added to the first mixture to make a soft material , pass through a 24-mesh sieve for granulation, dry the prepared granules at 60°C until the wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com