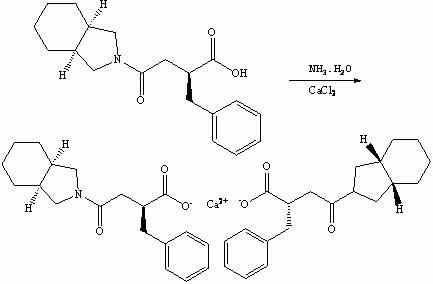
Preparation method of mitiglinide calcium
A technology of mitiglinide calcium and benzylsuccinic acid, which is applied in the field of preparation of drug mitiglinide calcium, can solve the problems of low chiral separation and waste, and achieve the effect of high chiral separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
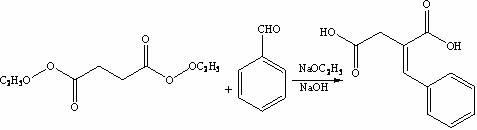
[0029] The synthesis of step 1, benzylidene succinic acid
[0030] Under stirring, add sodium metal (1.7 g, 0.072 mol) into absolute ethanol (50 mL), under the protection of argon, heat and stir until the solution refluxes, and the reflux state is maintained for 50 minutes, and benzaldehyde ( 23 mL, 0.183 mol), then diethyl succinate (50 mL, 0.275 mol) was added dropwise, and the reaction was continued to stir for 2.5 hours. LC-Ms detection showed that the proportion of raw material benzaldehyde decreased slowly. After cooling to room temperature, 55wt .% NaOH aqueous solution to adjust the pH ≥ 13.0, then heated to reflux for 3 hours, cooled to room temperature, kept the temperature of the reaction solution < 25 °C, adjusted the pH ≤ 2.0 with concentrated hydrochloric acid, filtered, and recrystallized THF at low temperature, yield: 81.3%;
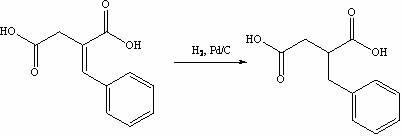
[0031] The synthesis of step 2, benzylsuccinic acid
[0032] Put benzylidene succinic acid (23.7 g, 0.114 mol) into the reactor, then a...
Embodiment 2
[0042] Similar to the experimental method of step 1 to step 6 of Example 1, except that in step 3, (R)-1-phenylethylamine (61.4 g, 0.359 mol) is used to replace (R)-1-phenylethylamine, other reaction operations The homogeneous phase is similar, and the synthetic yield of this step is: 87.3%.
Embodiment 3
[0044] Similar to the experimental method of step 1 to step 6 of Example 1, except that in step 3, (R)-1-phenyl-2-p-tolylethylamine (90.2 g, 0.374 mol) was used to replace (R)-1- Phenylethylamine, other reaction operations are similar, and the synthetic yield of this step is: 83.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com