Mitiglinide calcium dispersible tablet and preparation method thereof
A technology of mitiglinide calcium and dispersible tablets, which is applied in the field of dispersible tablets, can solve problems such as weight gain and patient hunger, and achieve the effects of short duration of action, fast onset of action, and good hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
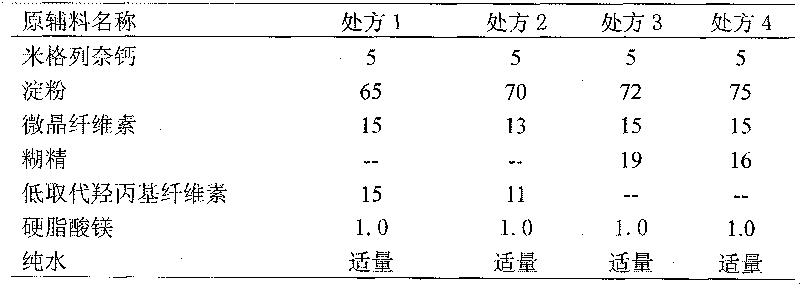
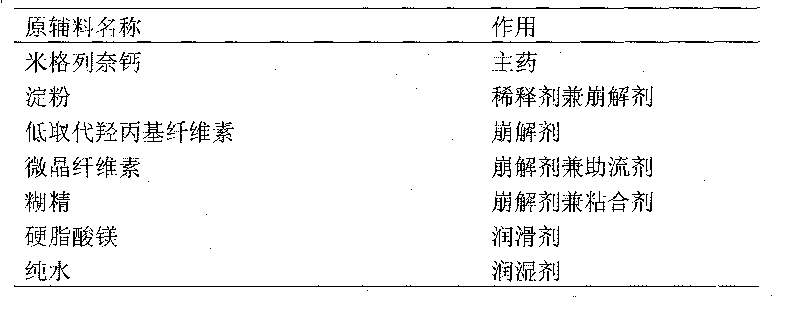
[0017] (1) 5 parts of mitaglinide calcium, 75 parts of starch, 15 parts of microcrystalline cellulose, 16 parts of dextrin, and 1.0 part of magnesium stearate (external) were pulverized through a 100-mesh sieve, and set aside;
[0018] (2) The mitiglinide calcium of the prescribed amount is fully mixed with microcrystalline cellulose and starch by an equivalent incremental method;
[0019] (3) Add the dextrin and magnesium stearate added in the prescription amount into the mixer together, and dry mix for 30 minutes;
[0020] (4) Add pure water to the mixed raw and auxiliary materials, mix to make a soft material, and granulate with a 30-mesh screen;
[0021] (5) Dry the granules in an electric oven at 45°C for 4.5 hours;
[0022] (6) Add the supplementary materials added to the prescription amount into the dry granules, granulate with a 30-mesh sieve, and mix evenly;
[0023] (7) Tablet packaging.
Embodiment 2
[0025] (1) 4 parts of mitaglinide calcium, 60 parts of starch, 12 parts of microcrystalline cellulose, 12.8 parts of dextrin, and 0.8 part of magnesium stearate (external) were pulverized through a 100-mesh sieve, and set aside;
[0026] (2) The mitiglinide calcium of the prescribed amount is fully mixed with microcrystalline cellulose and starch by an equivalent incremental method;
[0027] (3) Add the dextrin and magnesium stearate added in the prescription amount into the mixer together, and dry mix for 20 minutes;
[0028] (4) Add pure water to the mixed raw and auxiliary materials, mix to make a soft material, and granulate with a 30-mesh screen;
[0029] (5) Dry the particles in a 40°C electric oven for 4 hours;
[0030] (6) Add the supplementary materials added to the prescription amount into the dry granules, granulate with a 30-mesh sieve, and mix evenly;
[0031] (7) Tablet packaging.
Embodiment 3
[0033] (1) 6 parts of mitaglinide calcium, 90 parts of starch, 18 parts of microcrystalline cellulose, 19.2 parts of dextrin, and 1.2 parts of magnesium stearate (external) were pulverized through a 100-mesh sieve, and set aside;
[0034] (2) The mitiglinide calcium of the prescribed amount is fully mixed with microcrystalline cellulose and starch by an equivalent incremental method;
[0035] (3) Add the dextrin and magnesium stearate added in the prescription amount into the mixer together, and dry mix for 40 minutes;
[0036] (4) Add pure water to the mixed raw and auxiliary materials, mix to make a soft material, and granulate with a 30-mesh screen;
[0037] (5) Dry the particles in an electric oven at 50°C for 5 hours;
[0038] (6) Add the supplementary materials added to the prescription amount into the dry granules, granulate with a 30-mesh sieve, and mix evenly;
[0039] (7) Tablet packaging.
[0040] Prescription Basis
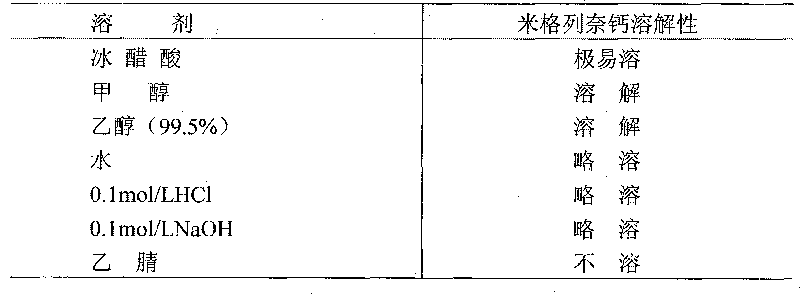
[0041] Based on the properties of mitiglinid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com