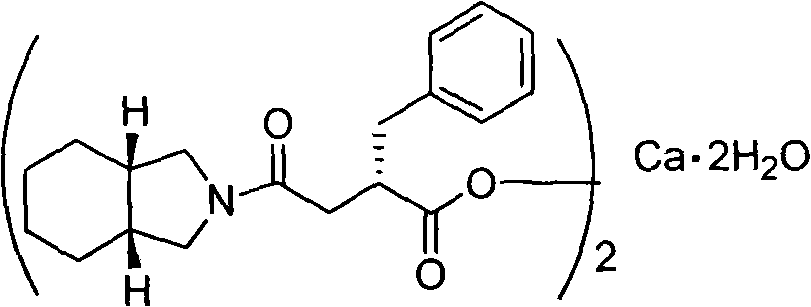
Method for preparing high purity mitiglinide calcium
A mitiglinide calcium and high-purity technology is applied in the field of preparation of high-purity mitiglinide calcium, can solve the problems of difficult purification, high cost, low yield and the like, and achieves strong controllability, low cost and high purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
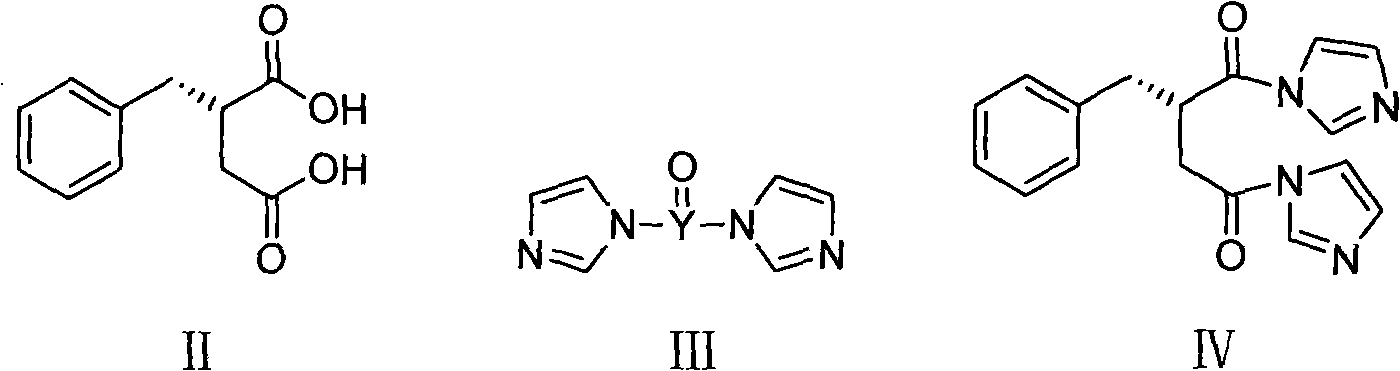
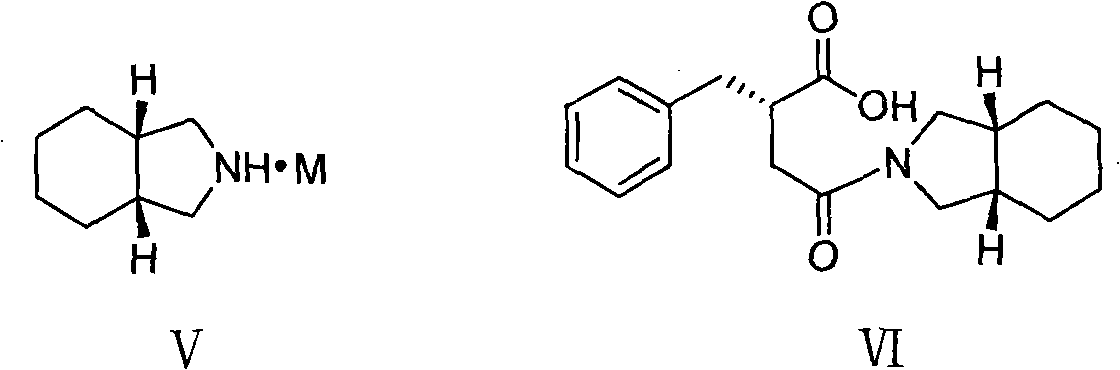
[0032] Under a nitrogen atmosphere, 1.5 L of dichloromethane and 65 g of carbonyldiimidazole were added to a 3 L reaction flask. Cool down to -5-0°C, add 62g of (S)-benzylsuccinic acid (compound of formula II), and keep stirring for 1 hour. Add 49g of perhydroisoindole hydrochloride, keep the temperature for 0.5 hours, and gradually raise the temperature of the reaction system to room temperature, and stir for 2 hours. Adjust to acidity with hydrochloric acid, separate the organic phase, and wash with distilled water. The organic phase was washed with saturated sodium bicarbonate. The aqueous phase was separated, adjusted to acidity with concentrated hydrochloric acid, extracted with dichloromethane, the organic phase was dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain a yellow oil. Add 150ml 95% ethanol and 300ml distilled water, and stir well. Adjust alkalinity with 2M sodium hydroxide solution and stir for 0.5 hours. A mixed solution of 55 g...
Embodiment 2
[0034] Under a nitrogen atmosphere, 1.5 L of dichloromethane and 73 g of sulfoxide diimidazole were added to a 3 L reaction flask. Cool down to -5-0°C, add 62g of (S)-benzylsuccinic acid (compound of formula II), and keep stirring for 1 hour. Add 49g of perhydroisoindole hydrochloride, keep the temperature for 0.5 hours, and gradually raise the temperature of the reaction system to room temperature, and stir for 2 hours. Adjust to acidity with hydrochloric acid, separate the organic phase, and wash with distilled water. The organic phase was washed with saturated sodium bicarbonate. The aqueous phase was separated, adjusted to acidity with concentrated hydrochloric acid, extracted with dichloromethane, the organic phase was dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain a yellow oil. Add 150ml 95% ethanol and 300ml distilled water, and stir well. Adjust alkalinity with 2M sodium hydroxide solution and stir for 0.5 hours. A mixed solution of 55...
Embodiment 3
[0036] Under a nitrogen atmosphere, 1.5 L of N,N-dimethylformamide and 73 g of carbonyldiimidazole were added to a 3 L reaction flask. Cool down to 0-5°C, add 62g of (S)-benzylsuccinic acid (compound of formula II), and keep stirring for 1 hour. Add 49g of perhydroisoindole hydrochloride, keep the temperature for 0.5 hours, and gradually raise the temperature of the reaction system to room temperature, and stir for 2 hours. The reaction solution was poured into distilled water, extracted with dichloromethane, washed with hydrochloric acid until acidic, washed with distilled water, and then washed with saturated sodium bicarbonate. The aqueous phase was separated, adjusted to acidity with concentrated hydrochloric acid, extracted with dichloromethane, the organic phase was dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain a yellow oil. Add 150ml 95% ethanol and 300ml distilled water, and stir well. Adjust alkalinity with 2M sodium hydroxide solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com