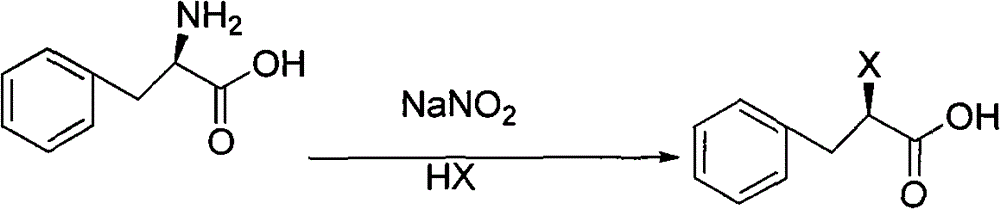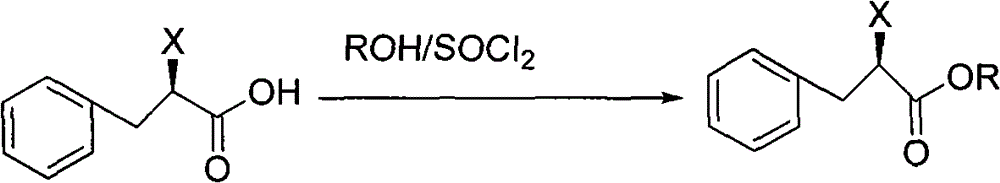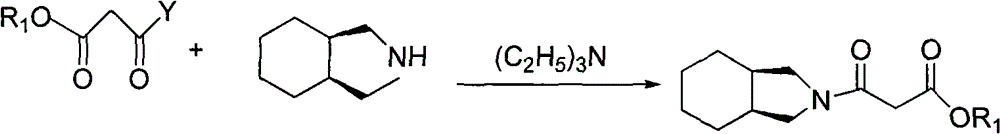Preparation method of mitiglinide calcium
A technology of mitiglinide calcium and compound is applied in the field of preparation of mitiglinide calcium, a medicine for treating type II diabetes, and can solve the problem that the specificity of chiral reduction products is not particularly strong, the resolution yield is low, and the by-products are many. and other problems, to avoid the problem of location selectivity, reduce production costs, and avoid high costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1, (R)-2-chlorophenylpropionic acid
[0048] In a 2000ml reaction flask, add 1500ml of 3N hydrochloric acid, add 165g (1.0mol) of L-phenylalanine under ice-salt bath cooling, stir, add 76g of sodium nitrite (1.1mol) aqueous solution dropwise below 5°C, and continue to control the temperature React for 2 hours, react overnight at room temperature, then add ethyl acetate to extract twice, add appropriate amount of anhydrous sodium sulfate to dry. Ethyl acetate was distilled off under reduced pressure to obtain about 180 g of slightly yellow residue with a yield of 97.8% (directly to the next step reaction). 1 H-NMR (400MHz, CDCl 3 )δ: 3.05 (1H, dd), 3.40 (1H, dd), 4.51 (1H, t), 7.26-7.38 (5H, m).
[0049] Preparation of (R)-2-chlorophenylpropionic acid methyl ester
[0050] Add 1000ml of anhydrous methanol to the oily substance from the previous step to dissolve, then add 131g (1.1mol) of thionyl chloride at below 10°C, slowly raise the te...
Embodiment 2
[0055] Embodiment 2, the preparation of 3-(2-cis perhydroisoindolyl)-3-oxopropionic acid ethyl ester
[0056] Add 125g (1.0mol) of cis-perhydroisoindole into 600ml of dichloromethane, add 111g (1.1mol) of triethylamine, control the temperature below 20°C, and slowly add 150g (1.0mol) of monoethyl malonate chloride dropwise. mol), the dropwise addition was completed and the reaction was continued for 2 hours, washed with water, dried over anhydrous sodium sulfate, filtered and concentrated to obtain 228 g of a colorless oily liquid, with a yield of 95.4% (directly proceed to the next step reaction).
Embodiment 3
[0057] Embodiment 3, the preparation of 2-benzyl-4-(2-cis perhydroisoindolyl)-4-oxobutanoic acid methyl ester
[0058] Add 228g (0.95mol) of ethyl 3-(cis-perhydroisoindolyl)-3-oxopropionate into 1300ml dimethyl sulfoxide, control the temperature below 15°C, and add 40g of 60% sodium hydride in batches (1.0mol), after the addition, continue to react for 1 hour, add 190g (0.96mol) of (R)-2-chlorophenylpropionic acid methyl ester, heat up to 55-60°C, react for 6 hours, point the plate to monitor the completion of the reaction, and cool to Below 20°C, add concentrated hydrochloric acid to adjust the pH to neutral, then raise the temperature to 150°C, react for 2 hours, cool down, then pour into ice water, extract twice with n-hexane, combine the organic layers, wash twice with water, dry and concentrate , 266 g of methyl 2-benzyl-4-(2-cis-perhydroisoindolyl)-4-oxobutyrate was obtained as a yellowish oily substance, with a yield of 87.3%. 1 H-NMR (CDCl 3 , 400M) δ: 1.28-1.54 (8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com