Preparation method of mitiglinide calcium
A technology of mitiglinide calcium and calcium salt, applied in the direction of organic chemistry, can solve the problems of rising process cost and low yield, avoiding the use of chiral catalysts or resolution reagents, simple reaction process and low price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
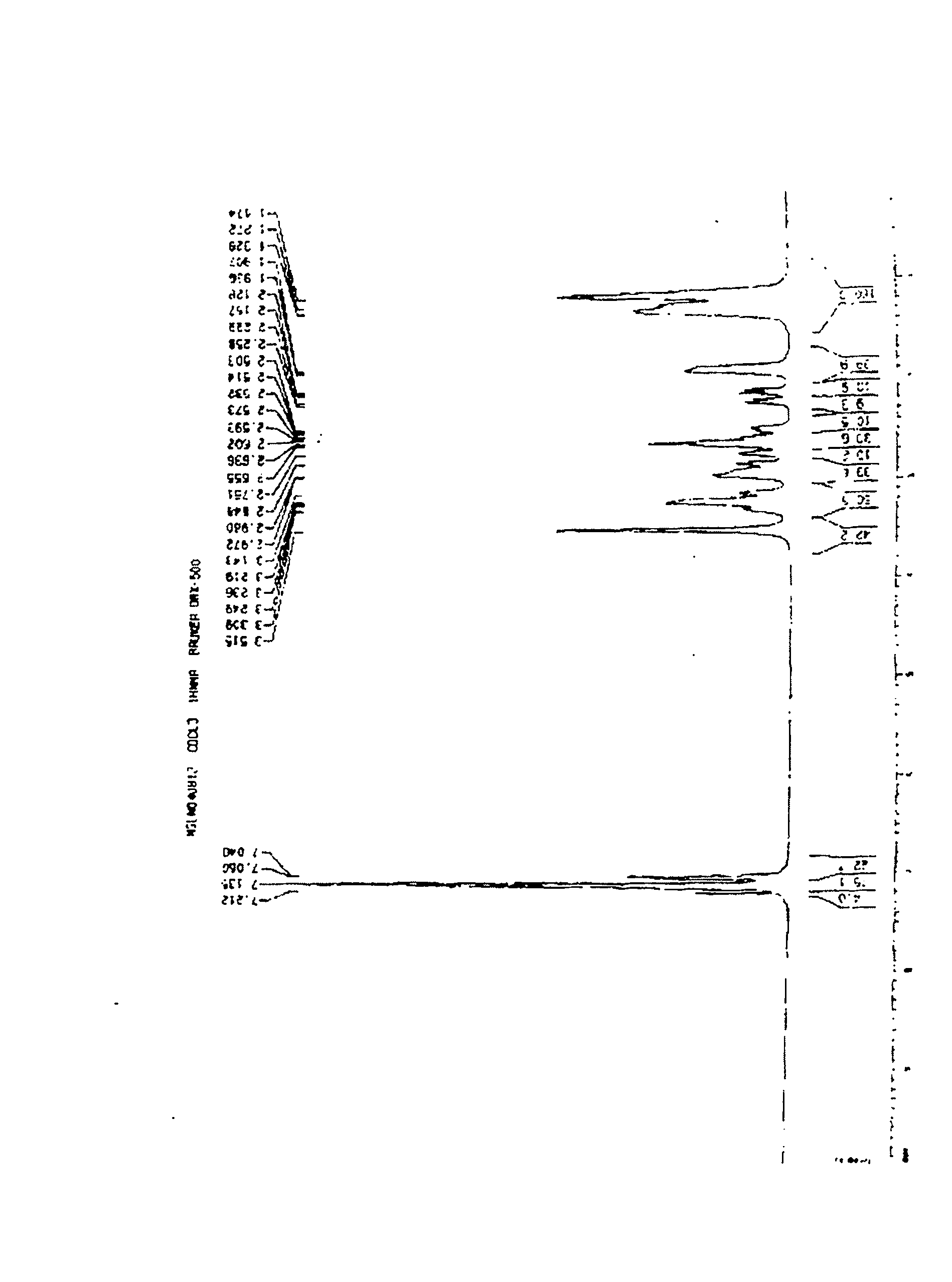
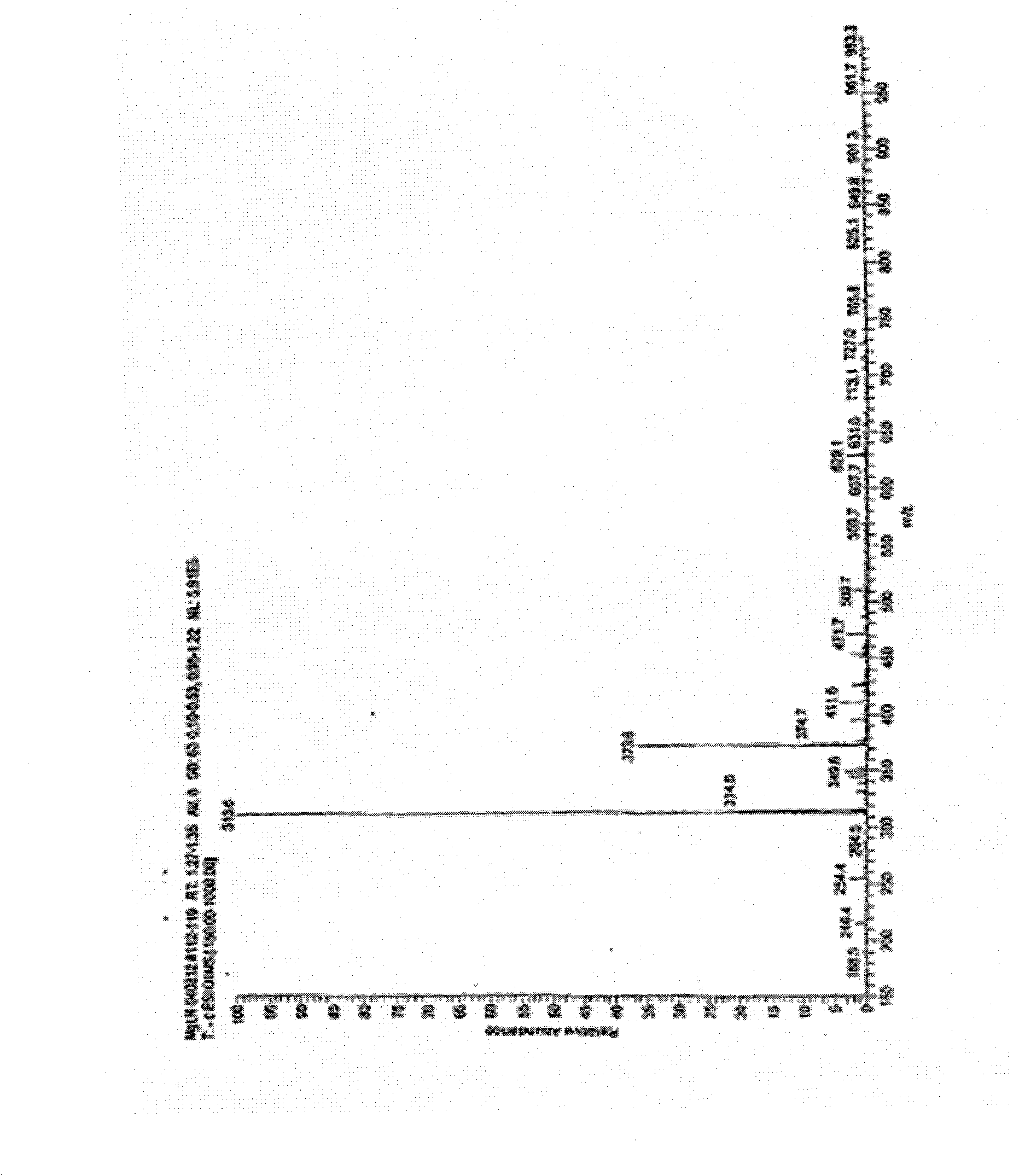
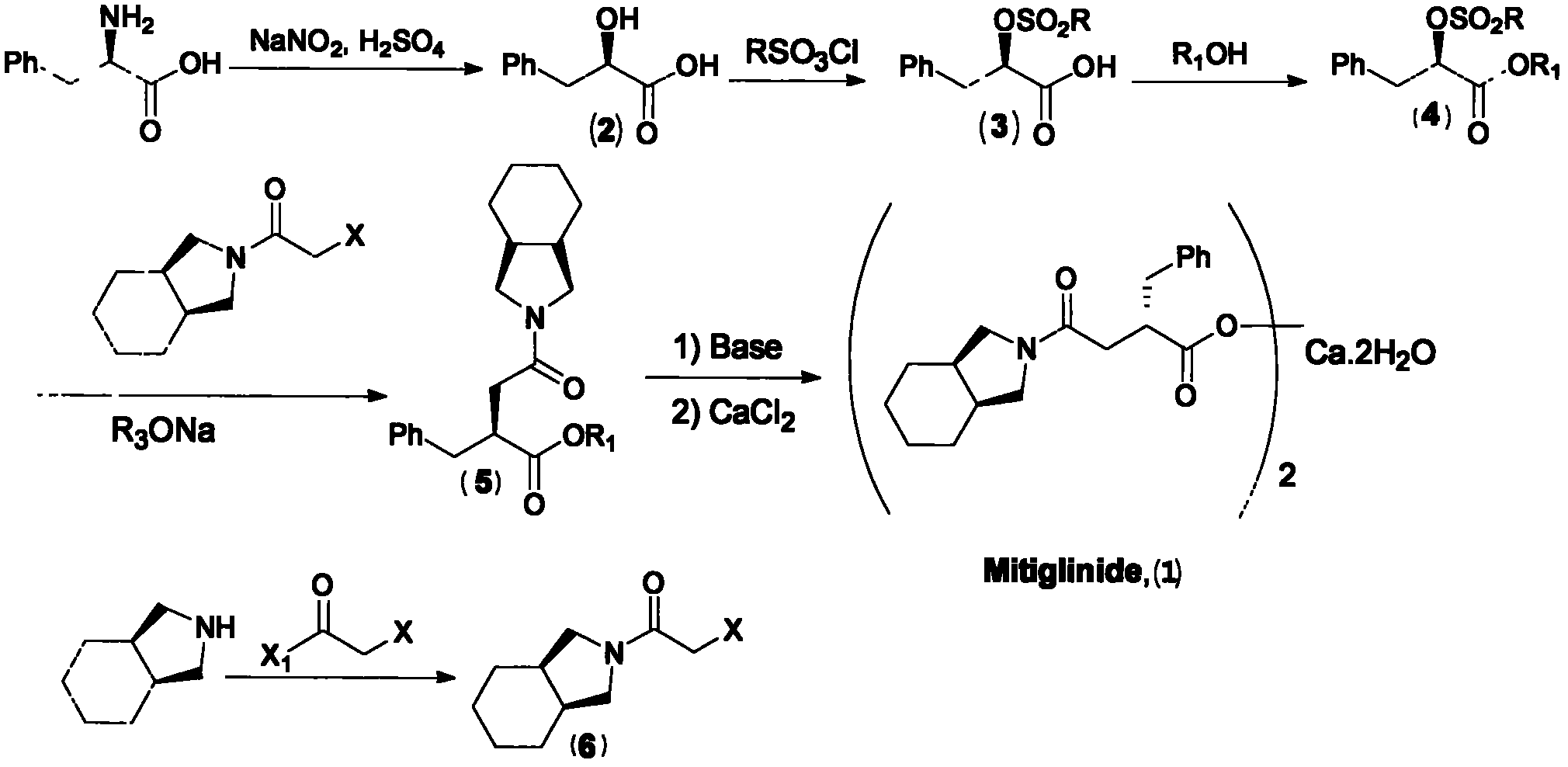
[0067] like Figure 1 to Figure 6 Shown, a kind of preparation method of preparing mitiglinide calcium, the steps are as follows:
[0068] Step 1: Hydrolyze D-phenylalanine under strong acid to obtain 2-hydroxyphenylpropionic acid
[0069] In a 5L reaction flask, add 3L of 6N sulfuric acid, add 330g (2mol) of D-phenylalanine under ice-salt bath cooling, stir, add 850g of 36% sodium nitrite aqueous solution dropwise at -5°C, and finish the reaction at room temperature. Overnight, the reaction mixture was extracted with ethyl acetate, combined and dried by adding an appropriate amount of anhydrous sodium sulfate. Ethyl acetate was distilled off under reduced pressure to obtain 320 g of light yellow solid with a yield of 95.0%.
[0070] Step 2: 2-hydroxyphenylpropionic acid protects the hydroxyl group with sulfonate under alkaline conditions to obtain hydroxyphenylpropionic acid sulfonate
[0071] In a 5L reaction flask, add 3L of pyridine, and dissolve the hydroxyphenylalanin...
Embodiment 2
[0081] Step 1: Hydrolyze D-phenylalanine under strong acid to obtain 2-hydroxyphenylpropionic acid
[0082] In a 5L reaction flask, add 3L of 6N sulfuric acid, add 330g (2mol) of D-phenylalanine under ice-salt bath cooling, stir, add 850g of 36% sodium nitrite aqueous solution dropwise below 5°C, and react overnight at room temperature after dropping. The reaction mixture was extracted with ethyl acetate, combined and dried by adding an appropriate amount of anhydrous sodium sulfate. Ethyl acetate was distilled off under reduced pressure to obtain 320 g of light yellow solid with a yield of 95.0%.
[0083] Step 2: 2-hydroxyphenylpropionic acid protects the hydroxyl group with sulfonate under alkaline conditions to obtain hydroxyphenylpropionic acid sulfonate
[0084] In a 5L reaction flask, add 3L of triethylamine, and dissolve the hydroxyphenylalanine obtained in the previous step. Slowly add 252g of p-methanesulfonyl chloride under 0°C, and react overnight at room temperatu...
Embodiment 3
[0094] Step 1: Hydrolyze D-phenylalanine under strong acid to obtain 2-hydroxyphenylpropionic acid
[0095]In a 5L reaction flask, add 3L of 6N sulfuric acid, add 330g (2mol) of D-phenylalanine under ice-salt bath cooling, stir, add 850g of 36% sodium nitrite aqueous solution dropwise below 0°C, and react overnight at room temperature after dropping. The reaction mixture was extracted with ethyl acetate, combined and dried by adding an appropriate amount of anhydrous sodium sulfate. Ethyl acetate was distilled off under reduced pressure to obtain 320 g of light yellow solid with a yield of 95.0%.
[0096] Step 2: 2-hydroxyphenylpropionic acid protects the hydroxyl group with sulfonate under alkaline conditions to obtain hydroxyphenylpropionic acid sulfonate
[0097] In a 5L reaction flask, add 3L of pyridine, and dissolve the hydroxyphenylalanine obtained in the previous step. Slowly add 400g of p-toluenesulfonyl chloride at a temperature below 0°C, and react overnight at roo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com