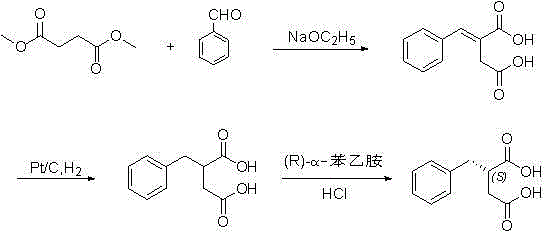
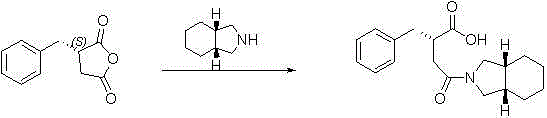
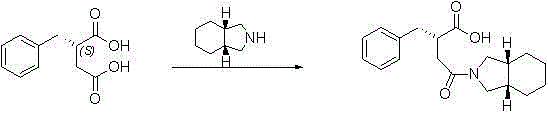Preparation method of (S)-2-benzylsuccinic acid
A technology of benzyl succinic acid and benzyl succinate, applied in the field of medicine, can solve problems such as waste and pollution, achieve the effects of improving economic benefits, reducing waste solvents and other pollutants, and protecting the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 catalyst blank test
[0024] will contain 100g 2-benzylsuccinic acid R -α-Phenylethylamine salt ( R / S =83 / 17) of the ethanol solution was concentrated under reduced pressure to recover ethanol, and then 500ml of water and 24g of sodium hydroxide were added to the remaining system and stirred to dissolve. The aqueous phase is extracted twice with dichloromethane, and the dichloromethane phase is combined to reclaim dichloromethane and ( R )-α-phenethylamine.
[0025] Heat the water phase to 80-90 oC Insulated for 12h, in the high performance liquid phase detection system ( R )-2-Benzylsuccinic acid and ( S )-2-benzylsuccinic acid ratio is 1:1, stop heating, cool down to 0-10 oC , add hydrochloric acid to the system to pH ≈ 1-2, at 0-10 o C for 2 hours, filter the system, and dry the solid. The yield is 95%.
[0026] The resulting 2-benzylsuccinic acid and R -α-Phenylethylamine is dissolved in ethanol by heating to reflux, and then crystallized o...
Embodiment 2
[0029] will contain 100g 2-benzylsuccinic acid R -α-Phenylethylamine salt ( R / S =85 / 15) of the ethanol solution was concentrated under reduced pressure to recover ethanol, and then 500ml of water and 35g of potassium hydroxide were added to the remaining system and stirred to dissolve. The aqueous phase is extracted twice with dichloromethane, and the dichloromethane phase is combined to reclaim dichloromethane and ( R )-α-phenethylamine.
[0030] Add 0.2g EDTA to the water phase and heat to 50-60 o C insulation for 4h, in the high performance liquid phase detection system ( R )-2-benzylsuccinic acid and (S)-2-benzylsuccinic acid ratio is 1:1, stop heating, cool down to 0-10 oC , add hydrochloric acid to the system to pH=1-2, at 0-10 o C for 2 hours, filter the system, and dry the solid. The yield is 96%.
[0031] The resulting 2-benzylsuccinic acid and R -α-Phenylethylamine salt crystallization in ethanol. Process by the method of embodiment 1, get S - Benzyl suc...
Embodiment 3
[0034] will contain 100g 2-benzylsuccinic acid R -α-Phenylethylamine salt ( R / S =85 / 15) of the ethanol solution was concentrated under reduced pressure to recover ethanol, and then 500ml of water and 80g of potassium carbonate were added to the remaining system and stirred to dissolve. The aqueous phase is extracted twice with dichloromethane, and the dichloromethane phase is combined to reclaim dichloromethane and ( R )-α-phenethylamine.
[0035] Add 0.4g EDTA to the water phase, then heat to 30-40 o C insulation for 3h, in the high performance liquid phase detection system ( R )-2-Benzylsuccinic acid and ( S )-2-benzylsuccinic acid ratio is 1:1, stop heating, cool down to 0-10 oC , add hydrochloric acid to the system to pH=1-2, at 0-10 o C for 2 hours, filter the system, and dry the solid. Yield 94.6%.
[0036] The obtained 2-benzylsuccinic acid and R-α-phenethylamine are salted and crystallized in ethanol. Process by the method of embodiment 1, get S - Benzyl s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com