Preparation method of mitiglinide calcium
A mitiglinide calcium and reaction technology, which is applied to the preparation field of the drug mitiglinide calcium, can solve the problems of many reaction steps, uneconomical, low yield and the like, saves the reaction time, is suitable for industrial production, and is easy to use. effect of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
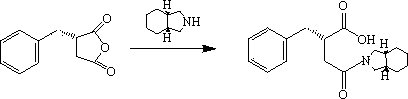
[0045] Ⅰ ( S Synthesis of )-2-benzylsuccinic acid
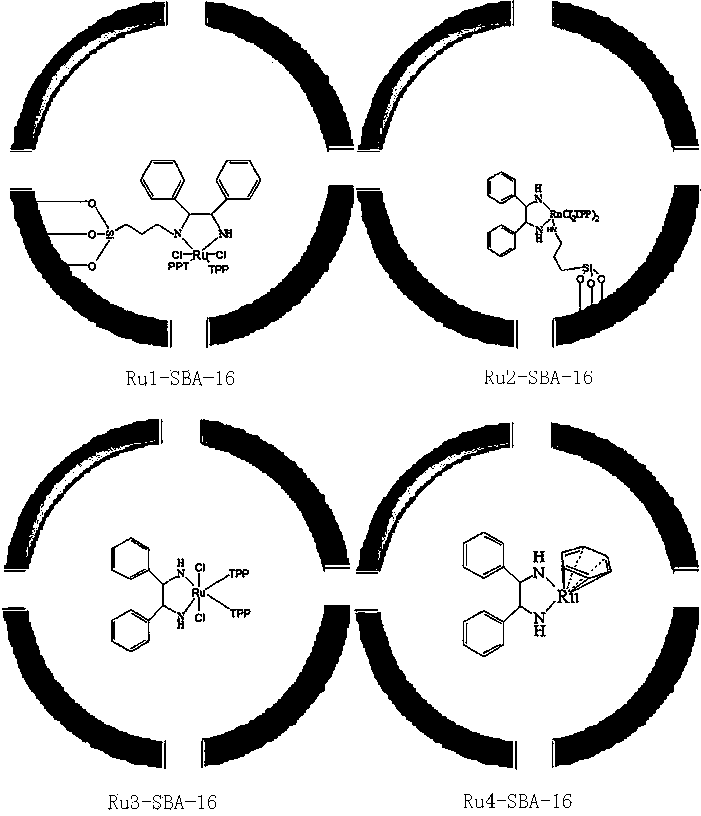
[0046] Under stirring, add 0.46 g (20 mmol) of sodium metal into absolute ethanol, under the protection of nitrogen, heat and stir until the solution refluxes, and mix 50 mmol of benzaldehyde and diethyl succinate at a ratio of 1:1 under reflux Add 50 mmol into the reactor and react for 4 h. After cooling to room temperature, add 0.5 g of the asymmetric catalyst Ru1-SBA-16. Hydrogen gas is introduced by bubbling at room temperature. After stirring for 6 h, filter. The catalyst is recovered, the filtrate is acidified with 1:1 hydrochloric acid (PH≤2.0), concentrated and crystallized, and recrystallized with ethyl acetate to obtain ( S )-2-benzylsuccinic acid 9.5 g, yield 91.3%.
[0047] II (2 S Synthesis of )-2-benzyl-3-(cis-hexahydroisoindole-2-carbonyl)propionic acid
[0048] Cool 50 mmol of cis-hexahydroisoindole in dichloromethane to 0°C, add 20 mmol of thionyl chloride in 30 mL of dichloromethane dropwise, stir at 0°C...
Embodiment 2
[0053] Ⅰ ( S Synthesis of )-2-benzylsuccinic acid
[0054] Under stirring, metal sodium 0.23 g (10 mmol) is added in absolute ethanol, under nitrogen protection, heat and stir until the solution is refluxed, 1: 1 mixed benzaldehyde 50 mmol and diethyl succinate Add 50 mmol into the reactor, react for 4 h, cool to room temperature, then add the asymmetric catalyst Ru2-SBA-16 (0.1 g), pass hydrogen gas at room temperature, stir and react for 6 h, then filter. The catalyst is recovered, the filtrate is acidified with 1:1 hydrochloric acid (PH≤2.0), concentrated and crystallized, and recrystallized with ethyl acetate to obtain ( S )-2-benzylsuccinic acid 8.6 g, yield 83%.
[0055] II (2 S Synthesis of )-2-benzyl-3-(cis-hexahydroisoindole-2-carbonyl)propionic acid
[0056] Cool 45 mmol of cis-hexahydroisoindole in dichloromethane to 4°C, add 25 mL of 18 mmol of thionyl chloride in chloroform dropwise, stir at 5°C for 1 h, then add ( S )-2-benzylsuccinic acid 8.6 g, continue to...
Embodiment 3
[0060] Ⅰ ( S Synthesis of )-2-benzylsuccinic acid
[0061] Under stirring, metal sodium 1.15g (50mmol) is added in absolute ethanol, under nitrogen protection, heat and stir until the solution is refluxed, under the reflux state, 1: 1 mixed benzaldehyde 50 mmol and diethyl succinate 50 Add mmol into the reactor, react for 4 h, cool to room temperature, then add the asymmetric catalyst Ru3-SBA-160.8 g, pass in hydrogen at room temperature, stir for 6 h, and then filter. Catalyst recovery, filtrate after 1: 1 hydrochloric acid acidification (PH≤2.0), concentrated crystallization, with ethyl acetate recrystallization, obtains ( S )-2-benzylsuccinic acid 10 g, yield 96%.
[0062] II (2 S Synthesis of )-2-benzyl-3-(cis-hexahydroisoindole-2-carbonyl)propionic acid
[0063] Cool 50 mmol of cis-hexahydroisoindole in dichloromethane to 0°C, add 20 mL of thionyl chloride 20 mmol in toluene dropwise, stir at -5°C for 1 h, then add ( S )-2-benzylsuccinic acid 10 g, continue to stir f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com