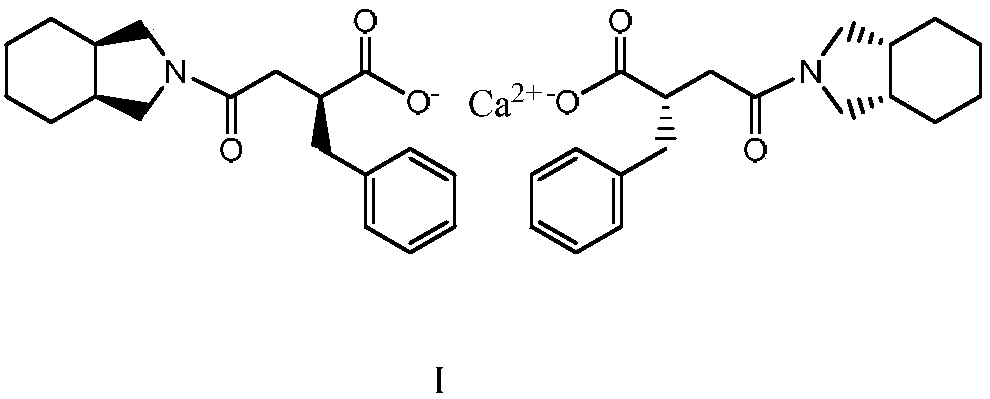
Method for preparing improved mitiglinide calcium
A technology of mitiglinide calcium and dichloromethane, which is applied in the field of preparation of mitiglinide calcium, can solve the problems of waste of raw materials and high production costs, and achieve the effects of reducing energy consumption, avoiding harm to the human body, and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
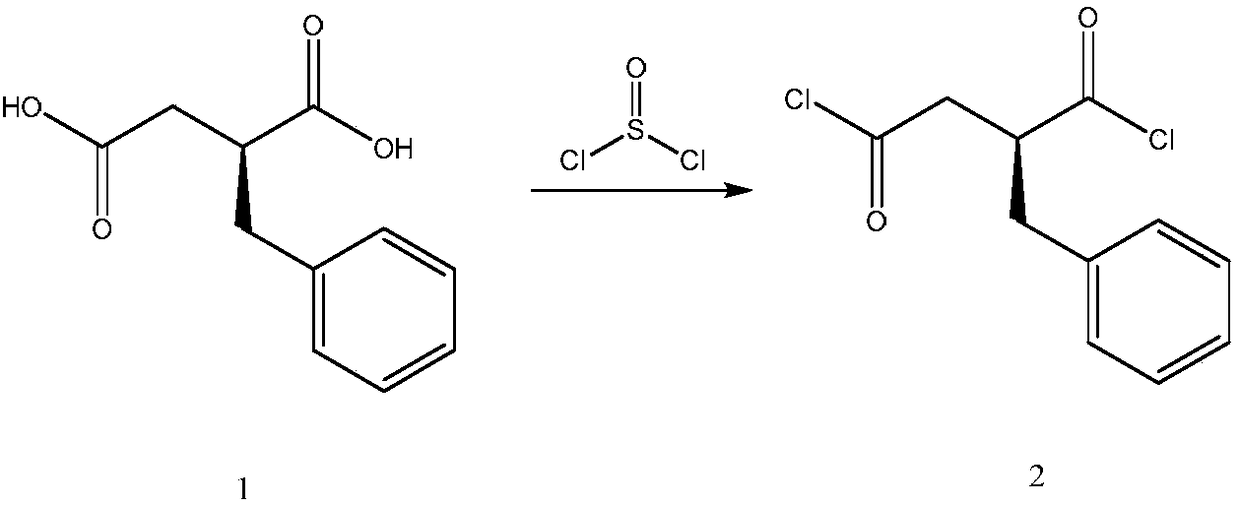
[0047] Step (1): Preparation of acid chloride
[0048] Add 41.6g of S-benzylsuccinic acid (0.2mol), 0.6g of triethylamine, and 220mL of dichloromethane into a 500mL three-neck flask, stir at room temperature for 20min, and heat to reflux. Weigh 52.0 g of thionyl chloride (0.44 mol) and slowly add it dropwise. After the drop is completed, reflux for 3 h while stirring, and cool to room temperature.
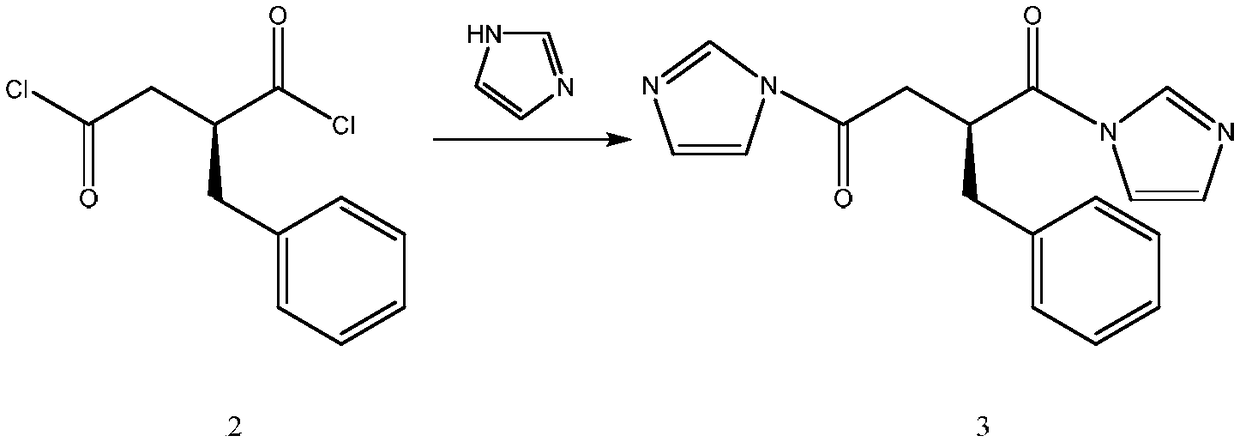
[0049] Step (2): Preparation of Active Amide
[0050] Add 28.8g of imidazole (0.42mol), 81.2g of triethylamine (0.80mol), and 300mL of dichloromethane into a 1000mL three-neck flask, stir until completely dissolved, cool down to about -12°C in an ice-salt bath, and perform step (1) The middle reaction solution was transferred to the dropping funnel, and slowly added dropwise to the mixed solution of imidazole which had dropped to -12°C. After the drop was completed, the reaction was stirred for about 1 hour, and the temperature was lowered to below -12°C in an ice-salt bath.
[0...
Embodiment 2
[0056] Step (1): Preparation of acid chloride
[0057] Add 41.6g of S-benzylsuccinic acid (0.2mol), 0.6g of triethylamine, and 220mL of dichloromethane into a 500mL three-neck flask, stir at room temperature for 20min, and heat to reflux. Weigh 52.0 g of thionyl chloride (0.44 mol) and slowly add it dropwise. After the drop is completed, reflux for 3 h while stirring, and cool to room temperature.
[0058] Step (2): Preparation of Active Amide
[0059] Add 34.2g of imidazole (0.5mol), 81.2g of triethylamine (0.80mol), and 300mL of dichloromethane into a 1000mL three-neck flask, stir until completely dissolved, and cool down to about -8°C in an ice-salt bath. The middle reaction solution was transferred to the dropping funnel, and slowly added dropwise to the mixed solution of imidazole which had dropped to -8°C. After the drop was completed, the reaction was stirred for about 1 hour, and the temperature was lowered to below -8°C in an ice-salt bath.
[0060] Step (3): 2-(S)-...
Embodiment 3
[0065] Step (1): Preparation of acid chloride
[0066] Add 41.6g of S-benzylsuccinic acid (0.2mol), 0.6g of triethylamine, and 220mL of dichloromethane into a 500mL three-neck flask, stir at room temperature for 20min, and heat to reflux. Weigh 52.0 g of thionyl chloride (0.44 mol) and slowly add it dropwise. After the drop is completed, reflux for 3 h while stirring, and cool to room temperature.
[0067] Step (2): Preparation of Active Amide
[0068] Add 41.2g of imidazole (0.6mol), 81.2g of triethylamine (0.80mol), and 300mL of dichloromethane into a 1000mL three-neck flask, stir until completely dissolved, cool down to about -4°C in an ice-salt bath, and perform step (1) The middle reaction solution was transferred to the dropping funnel, and slowly added dropwise to the mixed solution of imidazole which had dropped to -4°C. After the drop was completed, the reaction was stirred for about 1 hour, and the temperature was cooled to below -4°C in an ice-salt bath.
[0069] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com