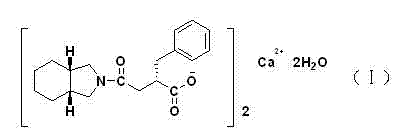
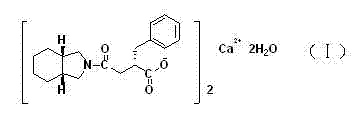
High purity mitiglinide calcium preparation method
A mitiglinide calcium and high-purity technology is applied in the field of preparation of high-purity mitiglinide calcium, can solve the problems of high reagent price, difficult liquid purification and storage, complicated process, etc., and achieves good reaction area selectivity, Ease of purification and preservation, and the effect of simplifying the synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: The preparation method of the high-purity mitiglinide calcium adopts the following specific process steps.
[0028] (1) (2S)-2-benzyl-3-(cis-hexahydroisoindole-2-carbonyl)propionic acid shown in the preparation formula (VII):
[0029]
[0030] Add 81.7g of N,N-carbonyldiimidazole and 500mL of dichloromethane solution into the reaction bottle, cool down to -10°C, add 50.0g (240mmoL) of S-benzylsuccinic acid, and keep the reaction at low temperature for 5 hours after addition; 39.2 g (242 mmoL) of cis-perhydroisoindole hydrochloride in 80 mL of dichloromethane was reacted at -10°C, spotted on TLC until the reaction was complete, and 6N HCl was added dropwise into the reaction bottle to adjust the pH to 1-2, and stirred for 1 After 1 hour, the organic phase was separated, and the organic phase was washed with distilled water to obtain a dichloromethane solution of (2S)-2-benzyl-3-(cis-hexahydroisoindole-2-carbonyl)propionic acid.
[0031] (2) (2S)-2-benzy...
Embodiment 2
[0042] Embodiment 2: The preparation method of the high-purity mitiglinide calcium adopts the following specific process steps.
[0043] (1) Preparation of (2S)-2-benzyl-3-(cis-hexahydroisoindole-2-carbonyl)propionic acid:
[0044] Add 16.4g of N,N-carbonyldiimidazole into 250mL of the reaction bottle, cool down the temperature of 100mL of dichloromethane solution to 0°C, add 10.0g (48mmoL) of S-benzylsuccinic acid, and keep the reaction at low temperature for 4.5 hours after adding 7.9g (49mmoL) of cis-perhydroisoindole hydrochloride in 30mL dichloromethane solution, react at 0°C, spot on TLC until the reaction is complete, drop 6N HCl into the reaction bottle to adjust the pH to 1~2, and stir for 1 hour , separated the organic phase, and washed the organic phase with distilled water to obtain a dichloromethane solution of (2S)-2-benzyl-3-(cis-hexahydroisoindole-2-carbonyl)propionic acid.
[0045] (2) Preparation of benzyl (2S)-2-benzyl-3-(cis-hexahydroisoindole-2-carbonyl)p...
Embodiment 3
[0049] Example 3: The preparation method of the high-purity mitiglinide calcium is as follows.
[0050] (1) Preparation of (2S)-2-benzyl-3-(cis-hexahydroisoindole-2-carbonyl)propionic acid:
[0051] Add 9.8g of N,N-carbonyldiimidazole into the 250mL reaction bottle, cool down the 60mL ethyl acetate solution to 10°C, add 6.0g (29mmoL) of S-benzylsuccinic acid, keep the reaction at low temperature for 4 hours after the addition, add dropwise 4.8g (29mmoL) of cis-perhydroisoindole hydrochloride in 40mL ethyl acetate solution, react at 10°C, spot on TLC until the reaction is complete, drop 6N HCl into the reaction bottle to adjust the pH to 1~2, and stir for 1 hour , separated the organic phase, and washed the organic phase with distilled water to obtain a dichloromethane solution of (2S)-2-benzyl-3-(cis-hexahydroisoindole-2-carbonyl)propionic acid.
[0052] Step (2)-step (5) adopts the ratio of raw materials, process conditions and steps described in Example 1.
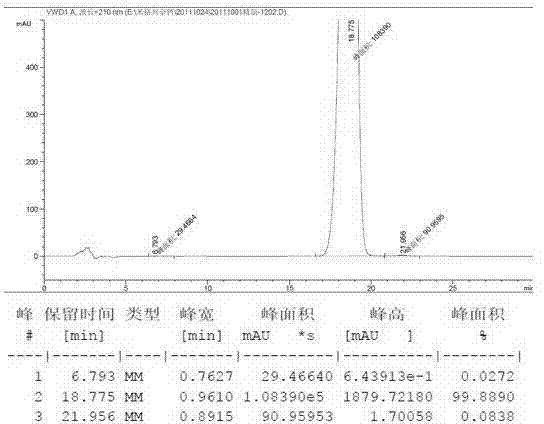
[0053] After r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com