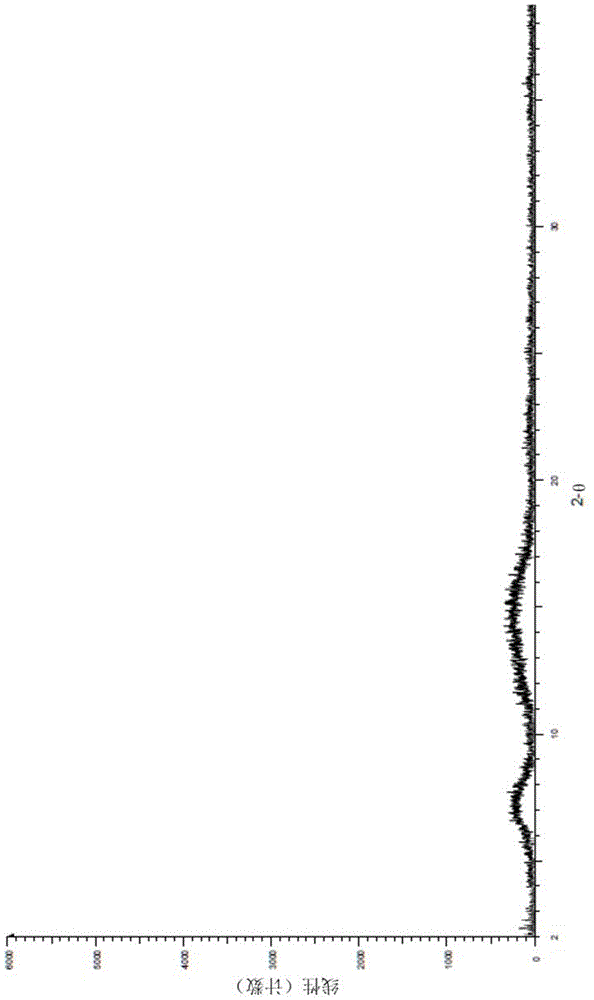I type crystal of L-alanine-(14-rubescensin A)-ester trifluoroacetate, and preparation method thereof
A technology of ester trifluoroacetate, oridonin, applied in the field of type I crystallization and preparation of L-alanine-(14-oridonin) ester trifluoroacetate, can solve the problem of Problems such as poor product stability, difficult intravenous administration, and poor fluidity have been achieved to achieve the effects of stable production process, repeatable and controllable production process, and good crystal form stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of L-alanine-(14-oridonin A) ester trifluoroacetate
[0040] Step 1: Preparation of N-Boc-L-alanine-(14-oridonin A) ester
[0041] Suspend 150g (0.41mol) oridonin (II), 195g (1.04mol) Boc-L-Ala in 1.35kg dichloromethane, cool in an ice bath to 0-5°C, add 213g (1.04mol) DCC, After stirring for 0.5h, the ice bath was removed, stirred at room temperature for 5h, and TLC confirmed that the reaction of the raw materials was complete (dichloromethane:methanol=10:1, raw material R f = 0.4, product R f = 0.6). The reaction solution was cooled to 0° C. and stood for 2 h, filtered, and the filter cake was washed with dichloromethane (300 g×3). The organic layers were combined and concentrated to obtain a white solid. Flash silica gel column chromatography (dichloromethane:methanol=100:1~60:1; v / v), collect product components, concentrate to dryness under reduced pressure to obtain about 140~165g of white solid powder. Add 600g of isopropyl ether to t...
Embodiment 2
[0044] Embodiment 2: Determination of the crystal form of the sample of Example 1
[0045] The solid sample X-ray powder diffraction pattern that makes in embodiment 1 sees image 3 , showing the characteristic absorption peak of the amorphous form, the DSC spectrum is shown in 4, and no melting characteristic absorption peak is seen below 300 ° C, so it is determined that the product is an amorphous solid.
Embodiment 3
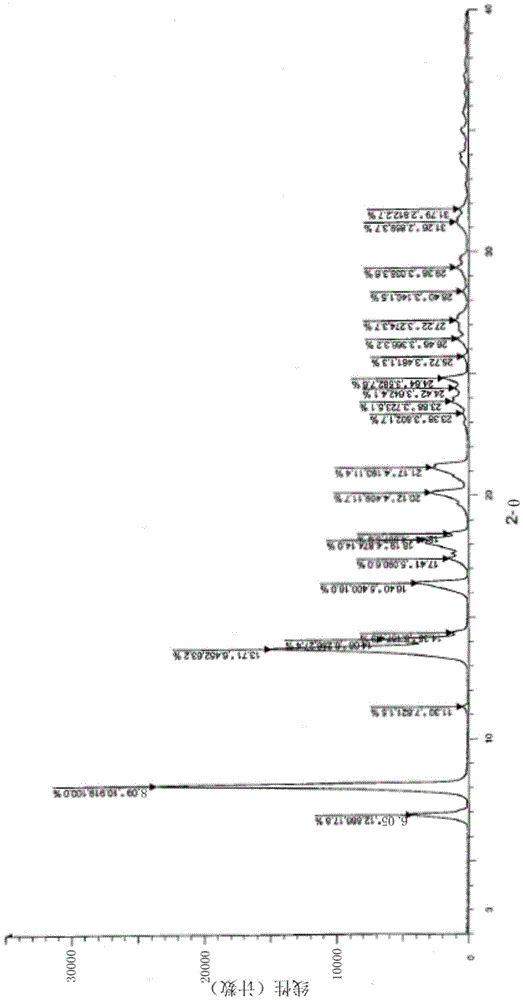
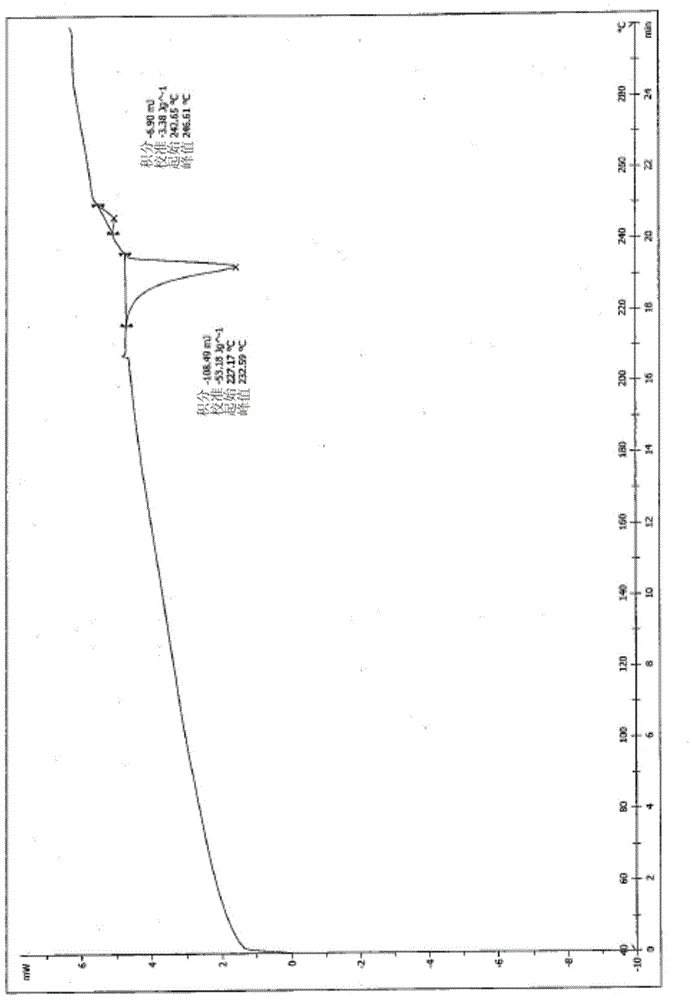
[0047] Weigh 500mg of L-alanine-(14-oridonin) ester trifluoroacetate amorphous solid in a 25ml round bottom flask, add 5ml of acetone, stir to dissolve, slowly add 10ml of isopropyl ether at room temperature, stir After crystallization for 2.5 hours, suction filtration, the filter cake was washed with a small amount of acetone / isopropyl ether (V / V=1 / 2) mixed solvent and dried to obtain 370 mg of white solid, yield 74.0%. The X-ray powder diffraction pattern of this crystalline sample is shown in figure 1 . The crystallization is at about 6.85 (12.89), 8.09 (10.92), 11.30 (7.82), 13.71 (6.45), 14.08 (6.29), 14.35 (6.17), 16.40 (5.40), 17.41 (5.09), 18.19 (4.87), 18.44 (4.81), 20.12(4.41), 21.17(4.19), 23.38(3.80), 23.88(3.72), 24.42(3.64), 24.84(3.58), 25.72(3.46), 26.46(3.37), 27.22(3.27), 28.40 There are characteristic peaks at (3.14), 29.38(3.04), 31.26(2.86) and 31.79(2.81). DSC spectrum see figure 2 , with a sharp melting endothermic peak at 232.59°C, this crystal for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com