A preparing method of a lenalidomide-nicotinamide eutectic composition
A technology of lenalidomide and nicotinamide, which is applied in the field of preparation of co-crystals of lenalidomide and nicotinamide, can solve problems such as insufficient enhancement effect and failure to meet the medicinal requirements of lenalidomide, and achieve Large solubility and dissolution rate, improved bioavailability and drug efficacy, and the effect of meeting pharmaceutical requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Weigh 1112.7 mg (4.3 mmol) of lenalidomide and 522.4 mg (4.3 mmol) of nicotinamide into a crystallizer, add 50 mL of methanol, mix and stir to form a suspension; keep stirring at 20°C for 3 hours; filter, filter The obtained solid is dried at room temperature (the temperature is 25° C.) to obtain a co-crystal of lenalidomide and nicotinamide.
[0045] The crystal structure of the obtained eutectic was determined by an APEX II CCD X-ray single crystal diffractometer. The test results show that the obtained crystal is a triclinic crystal system with a P-1 space group. The unit cell parameters are: unit cell volume Z=2, molecular formula: C19H19N5O4;
[0046] The crystallographic parameters are shown in Table 1:
[0047] Table 1
[0048]
[0049]
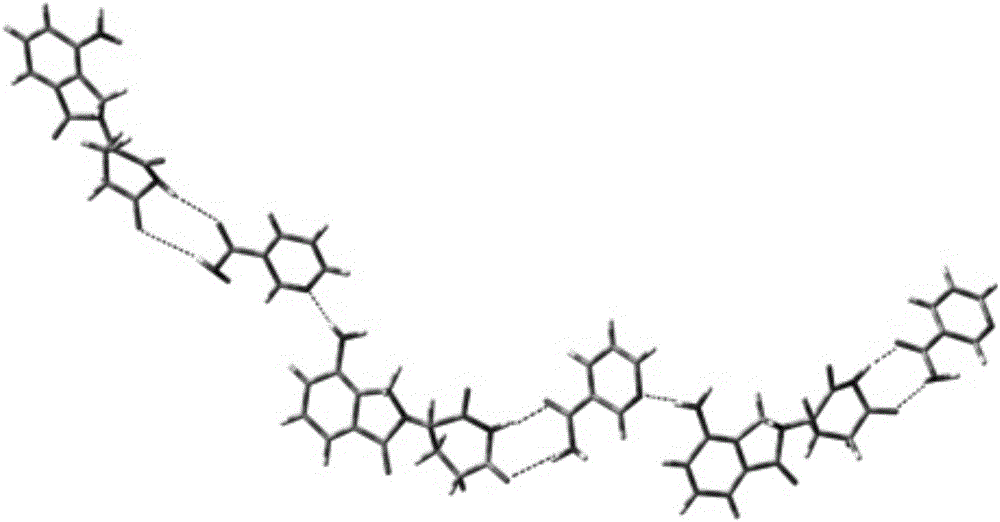
[0050] According to the measured data, draw the structure diagram of the co-crystal of lenalidomide and nicotinamide, such as figure 1 shown.
[0051] figure 1For the structural schematic diagram of the obtained ...
Embodiment 2
[0057] Weigh 2014.1 mg (7.8 mmol) of lenalidomide and 948.7 mg (7.8 mmol) of nicotinamide into a crystallizer, add 50 mL of methanol, mix and stir to form a suspension; keep stirring at 30 °C for 2.5 h; filter, The solid obtained by filtration is dried at room temperature (temperature is 25° C.) to obtain a co-crystal of lenalidomide and nicotinamide.
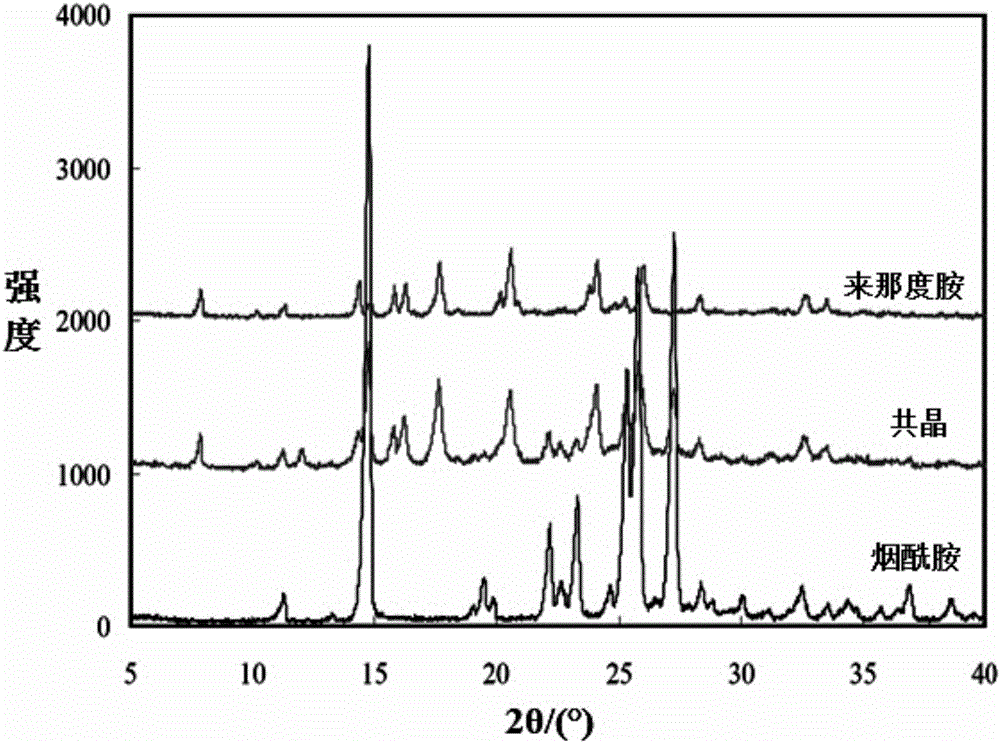
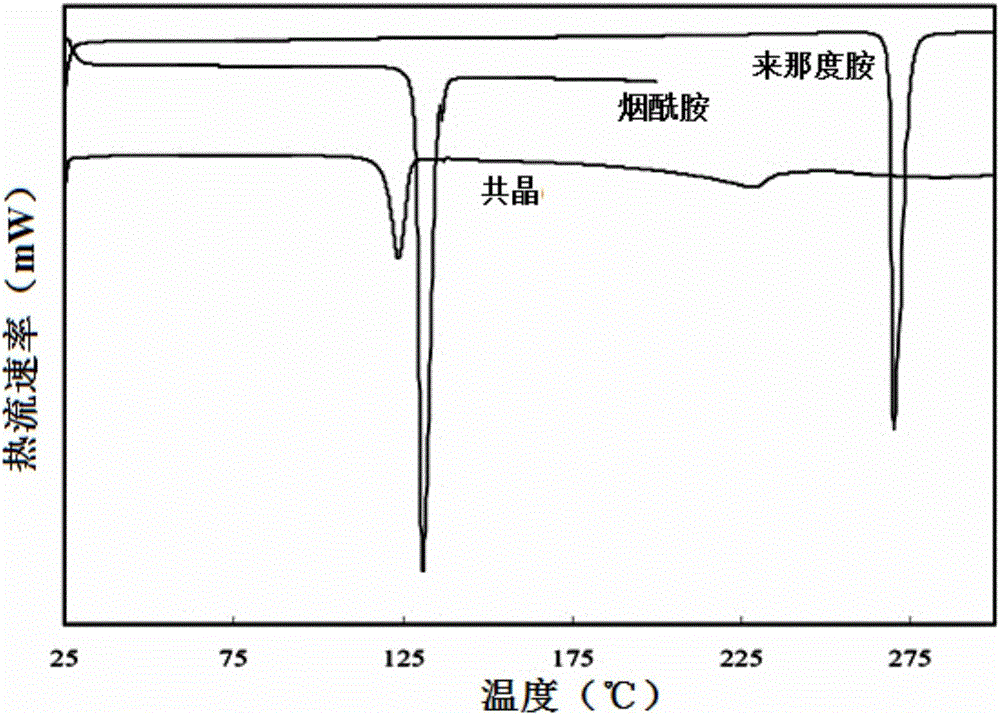
[0058] After analysis, the obtained solid product has figure 1 The structural schematic features shown, figure 2 The PXRD spectrum shown, image 3 The DSC spectral characteristics shown and Figure 4 The characteristics of the infrared spectrum shown indicate that the solid product obtained in this example is also the co-crystal of lenalidomide and nicotinamide of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com