Multiple crystal forms of S-Montelukast sodium and preparation method thereof
A technology of manidipine hydrochloride and manidipine, which is applied to the polymorphic form of S-manidipine hydrochloride and the field of preparation thereof, can solve the problems of low solubility of S-manidipine and the like, and achieves a production process. Stable, repeatable and controllable effects with good crystal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
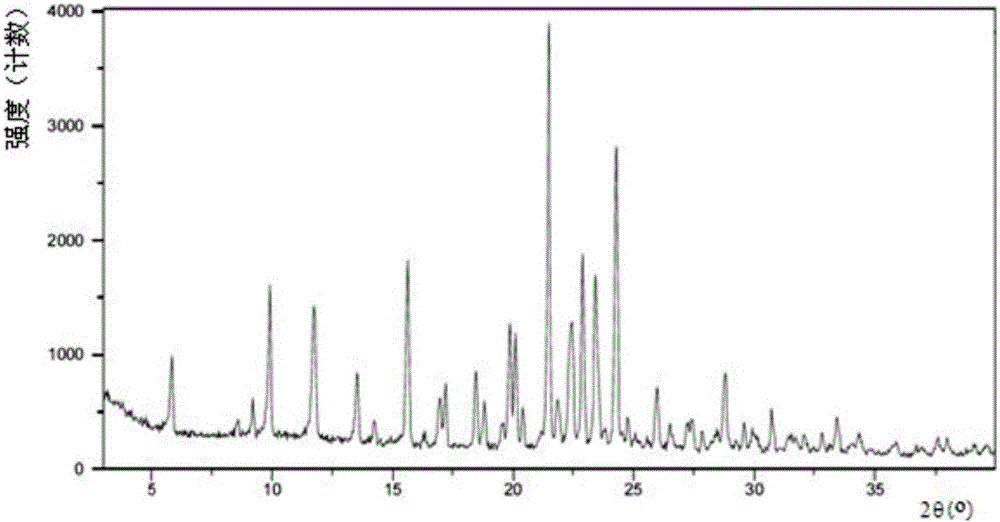
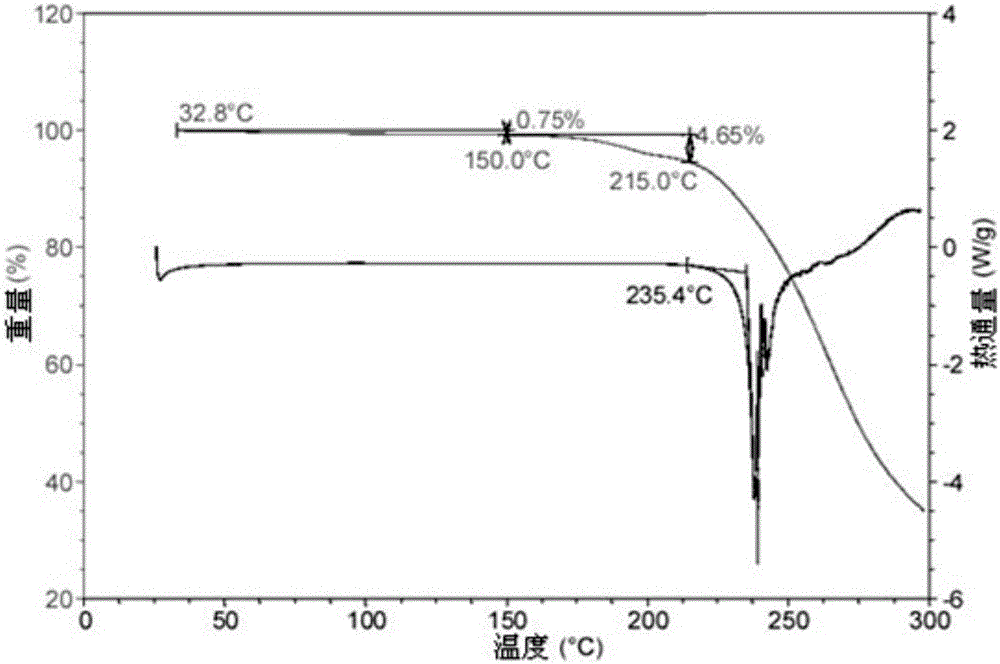
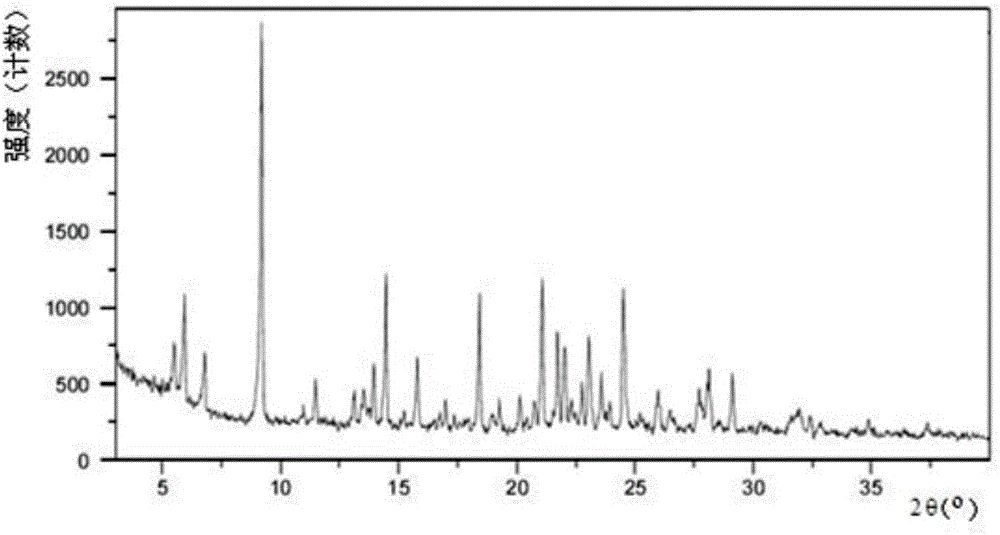
[0070] Mix 1 gram of S-manidipine free base (prepared according to Chinese patent application 201510870914.8) and 20 ml of acetone. Then, under stirring, 5 ml of 30% HCl / ethanol solution was added, and the stirring was continued until the solution was completely dissolved. The resulting clear solution was left to stand at room temperature for 8 hours for crystallization, and then filtered to obtain 0.77 g of S-manidipine hydrochloride. As detected by XRD, the crystals were at about 5.86, 8.56, 9.20, 9.88, 11.75, 13.52, 14.25, 15.61, 16.33, 16.96, 17.20, 18.46, 18.80, 19.55, 19.85, 20.08, 21.47, 21.84, 22.42, 22.428, 2 There are characteristic peaks at 24.27, 24.75, 25.95, 26.48, 27.23, 27.40, 27.83, 28.44, 28.77, 29.56, 29.90, and 30.70. Its X-ray diffraction pattern is shown in figure 1 , X-ray diffraction data are shown in Table 1 below. Its DSC spectrum is shown in figure 2 , There is a melting endothermic peak at 235.4°C. This crystal form is defined as II crystal fo...
Embodiment 2
[0076] Mix 1 gram of S-manidipine free base and 20 ml of ethyl acetate. Then, under stirring, 5 ml of 30% HCl / ethanol solution was added, and the stirring was continued until the solution was completely dissolved. The obtained clear solution was left to stand at room temperature for 8 hours for crystallization, and then filtered to obtain 0.81 g of S-manidipine hydrochloride. Its XRD and DSC patterns are researched and compared, and it is determined that the product is in the II crystal form.
Embodiment 3
[0078] Mix 1 gram of S-manidipine free base and 100 ml of methyl isobutyl ketone. Then, under stirring, 5 ml of 30% HCl / ethanol solution was added, and the stirring was continued until the solution was completely dissolved. The resulting clear solution was left to stand at room temperature for 8 hours for crystallization, and then filtered to obtain 0.67 g of S-manidipine hydrochloride. Its XRD and DSC patterns are researched and compared, and it is determined that the product is in the II crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com