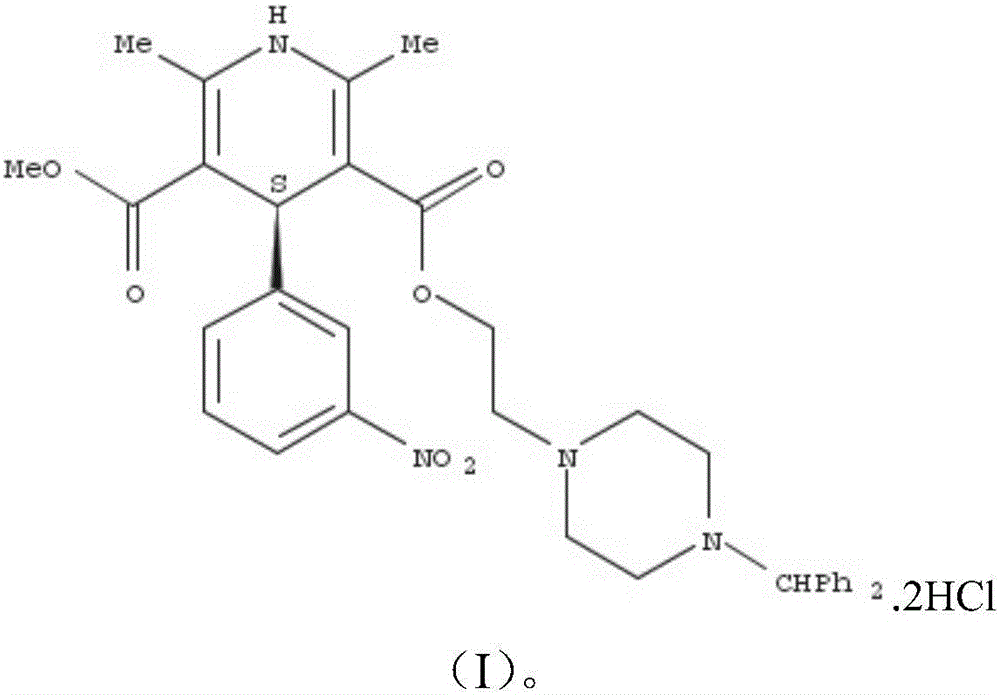
S-Manidipine hydrochloride crystal form I and preparation method thereof
A technology of manidipine hydrochloride, type I, which is applied in the field of pharmaceutical compositions containing it, can solve the problems of low solubility of S-manidipine and the like, and achieves repeatable and controllable production process, stable production process, and crystallinity. good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
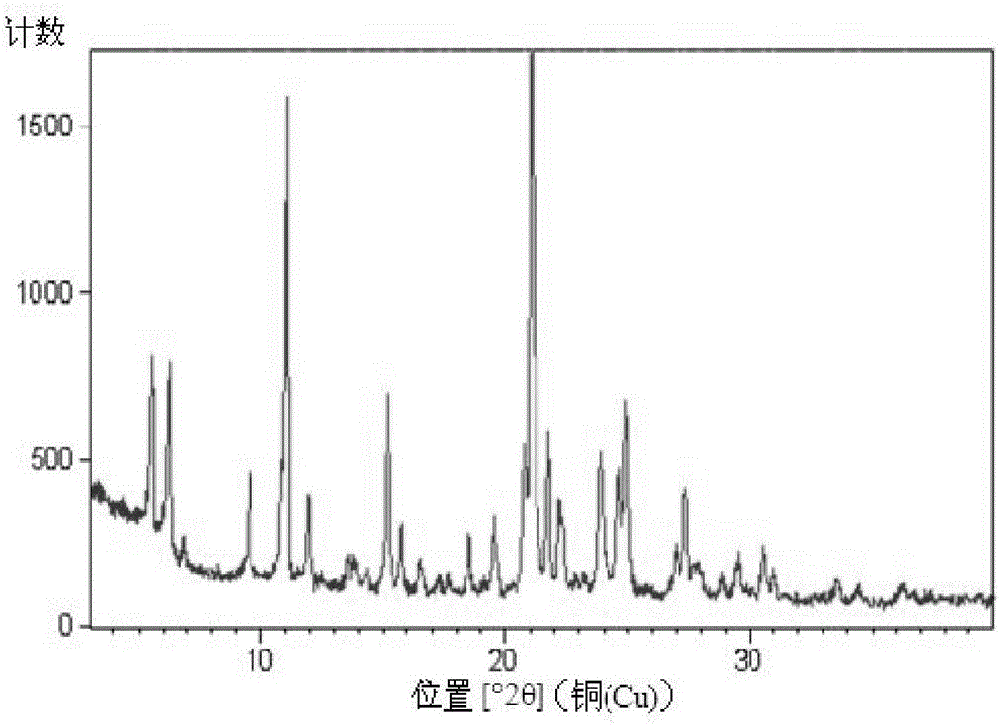
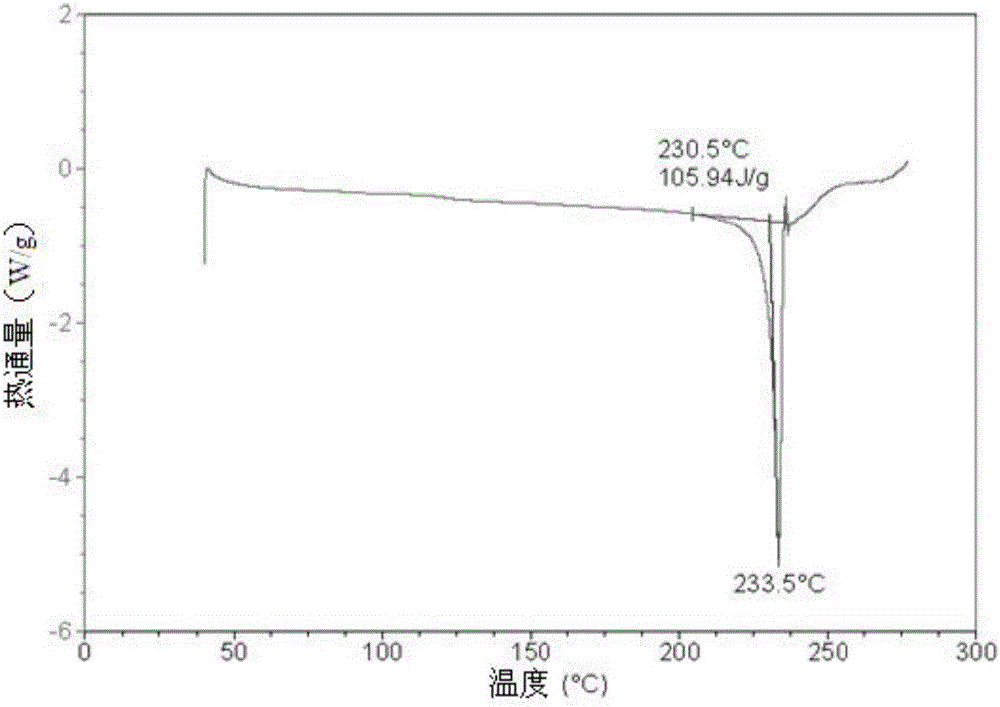
[0055] Mix 1 gram of S-manidipine free base (prepared according to Chinese patent application 201510870914.8) and 20 ml of absolute ethanol. Then, under stirring, 5 ml of 30% HCl / ethanol solution was added, and the stirring was continued until the solution was completely dissolved. The resulting clear solution was left to stand at room temperature for 8 hours for crystallization, and then filtered to obtain 0.82 g of S-manidipine hydrochloride. Detected by XRD, the crystallization is at about 5.54 (15.97), 6.23 (14.18), 9.56 (9.26), 11.05 (8.00), 11.92 (7.42), 15.16 (5.84), 15.78 (5.62), 18.51 (4.79), 19.54 ( 4.54),20.78(4.27),21.08(4.22),21.75(4.09),22.20(4.00),23.89(3.73),24.67(3.61),24.94(3.57),26.96(3.31),27.38(3.26),29.51( 3.03), there are characteristic peaks at 30.57 (2.92). Its X-ray diffraction pattern is shown in figure 1 , X-ray diffraction data are shown in Table 1 below. Its DSC spectrum is shown in figure 2 , There is a melting endothermic peak at 233.5°C. ...
Embodiment 2
[0059] Mix 1 g of S-manidipine free base and 10 ml of absolute ethanol. Then, under stirring, 5 ml of 30% HCl / ethanol solution was added, and the stirring was continued until the solution was completely dissolved. The resulting clear solution was left to stand at room temperature for 8 hours for crystallization, and then filtered to obtain 0.84 g of S-manidipine hydrochloride. Its XRD and DSC patterns are researched and compared, and it is confirmed that the product is I crystal form.
Embodiment 3
[0061] Mix 1 g of S-manidipine free base and 100 ml of absolute ethanol. Then, under stirring, 5 ml of 30% HCl / ethanol solution was added, and the stirring was continued until the solution was completely dissolved. The resulting clear solution was allowed to stand at room temperature for crystallization for 8 hours, and then filtered to obtain 0.65 g of S-manidipine hydrochloride. Its XRD and DSC patterns are researched and compared, and it is confirmed that the product is I crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com