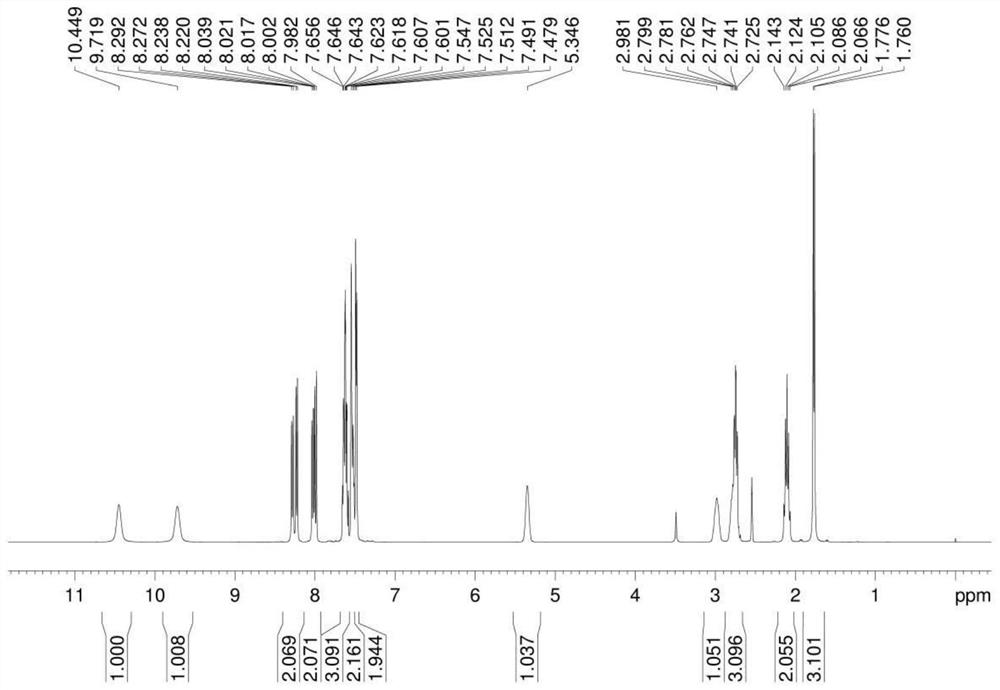
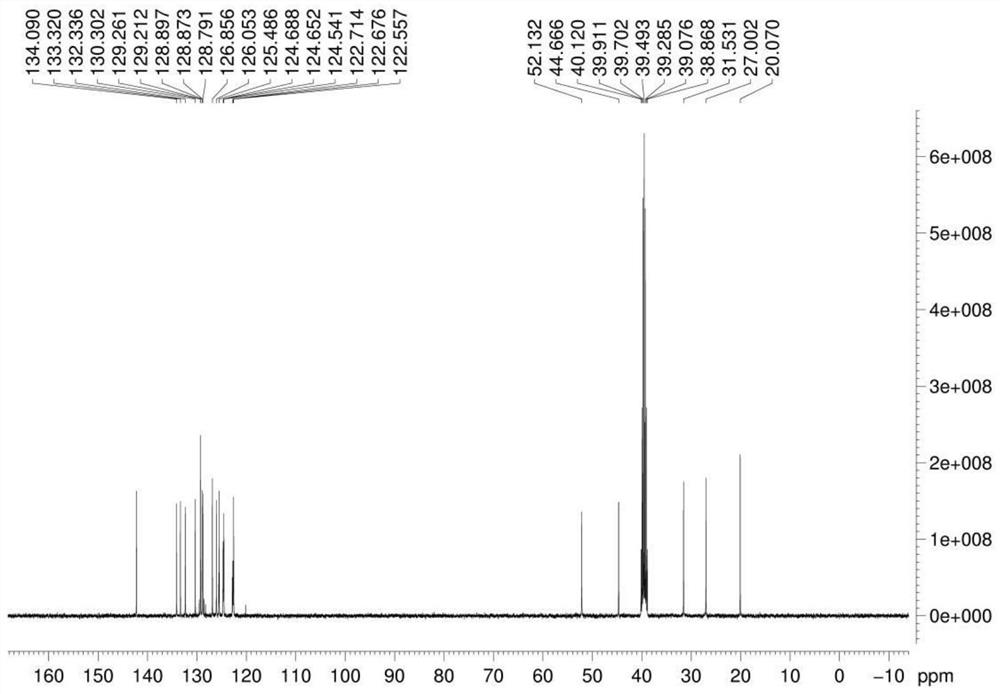
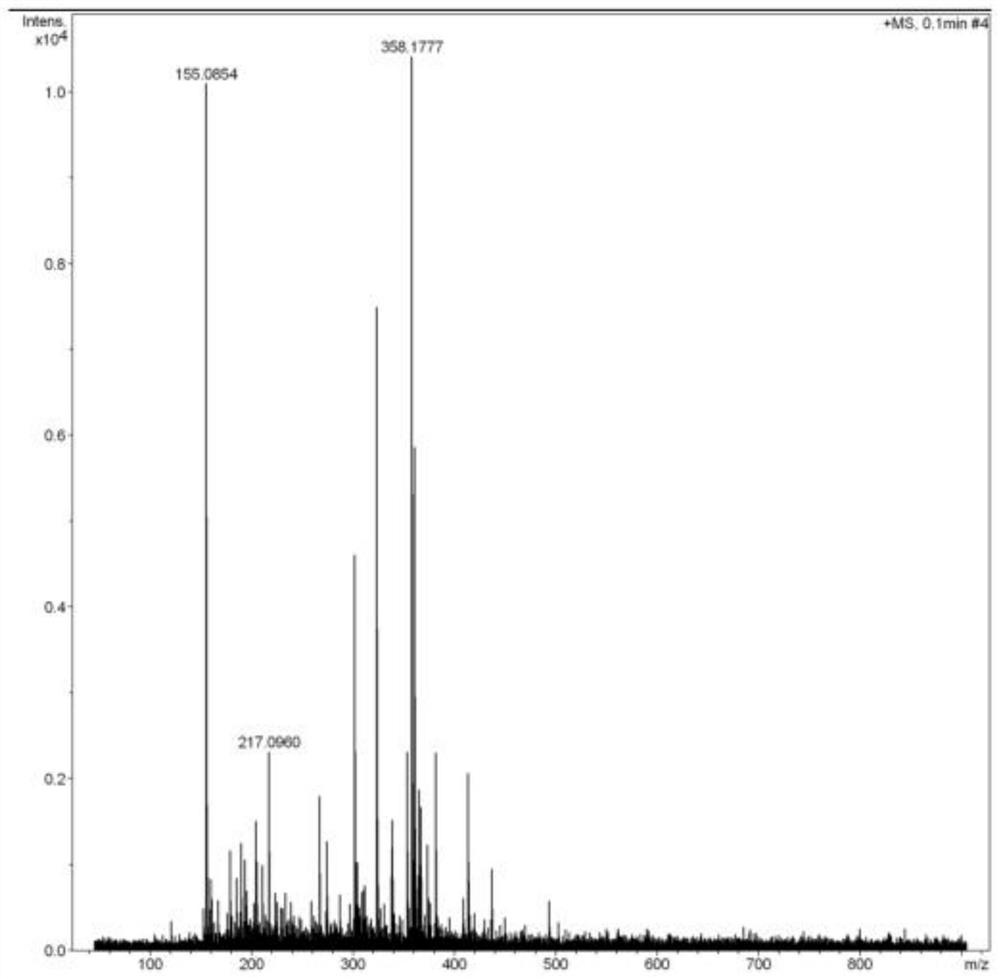
Preparation method of cinacalcet hydrochloride
A technology of cinacalcet hydrochloride and cinacalcet, which is applied in the field of drug synthesis, can solve the problems of large investment and high equipment requirements, achieve low production costs, meet medicinal requirements, and avoid hydrogenation reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of compound 5-(3-trifluoromethylbenzylidene) imidazoline-2,4-dione
[0038]Add 21.0g hydantoin, 34.8g m-trifluoromethylbenzaldehyde, 200mL water, 20mL absolute ethanol to a 500mL reaction bottle, heat and reflux at 100°C for 6h, and detect by TLC, after m-trifluoromethylbenzaldehyde basically disappears , stop heating, stir at room temperature for 10h, filter, wash the filter cake with a small amount of absolute ethanol, and dry under vacuum at 50°C (vacuum degree is -0.09MPa) to obtain compound 5-(3-trifluoromethylbenzylidene)imidazoline 43.5 g of -2,4-dione light yellow solid, yield 85.0% (yield calculated as m-trifluoromethylbenzaldehyde).
Embodiment 2
[0039] Example 2 Preparation of compound 2-oxo-trifluoromethylphenylpropionic acid
[0040] In the 500mL reaction bottle, add 5-(3-trifluoromethylbenzylidene) imidazoline-2,4-dione light yellow solid 43g, the mass percent concentration is 5% sodium hydroxide solution 200mL, in 105 Heat at reflux for 2 hours at ℃, after the reaction is complete, stop heating, cool down to room temperature, adjust the pH of the solution to 3 with hydrochloric acid with a mass percent concentration of 10% under ice bath conditions, stir and crystallize for 9 hours; filter with suction, wash the filter cake with a small amount of ethanol, 50 After vacuum drying at °C, 30.8 g of the compound 2-oxo-trifluoromethylphenylpropionic acid was obtained, with a yield of 75.1%.
Embodiment 3
[0041] Example 3 Preparation of compound (R)-3-(1-(naphthalene-1-yl)ethylamino)-1-(3-(trifluoromethyl)phenyl)propan-2,3-dione
[0042] Into a 500mL reaction flask, add 29.5g of 2-oxo-trifluoromethylphenylpropionic acid and 150mL of anhydrous tetrahydrofuran, stir for 30min, cool down to -10°C, add 22.1g / 14.7mL of oxalyl chloride dropwise, after addition, add N,N-Dimethylformamide 10mL, slowly warm up to 20°C, stir for 1h, then cool down to below 0°C, add (R)-1-(1-naphthyl)ethylamine solution in N-methylpyrrolidone dropwise Dissolve 82.2g [(R)-1-(1-naphthyl)ethylamine 21.0g in 100mL N-methylpyrrolidone, the mass percentage concentration is 17.0%], after dropping, keep stirring for 1h, add the reaction solution into 300mL purified water , add 200mL toluene, stir for 15min, then transfer to a 1000mL separatory funnel, let stand, separate the liquids, dry the organic phase with anhydrous sodium sulfate overnight, filter with suction, concentrate to get the compound (R)-3-(1-( Nap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com